Physico-Chemical Studies of
the Pvc K+ - Selective Membrane
COROIAN Ana1, PICĂ Elena Maria2*, GRECU Rodica1,
COSMA Viorica1, HOPÂRTEAN Elena1, HOPÂRTEAN Ioana1
1Institute of Chemistry Cluj-Napoca, Romania
2Technical University Cluj-Napoca, Romania
* Corresponding author, e-mail: empica@yahoo.com
Abstract
A plasticized ion-selective membrane based on PVC matrix which tricrezylphosphate (TCP) and containing K+ - ionophores (dibenzo-18-crown-6 and decyl-18-crown-6) was used to obtain a potentiometric potassium sensor.
The potassium selective membranes were characterized in terms of their electrochemical and physical properties, surface morphology and structural parameters. The a.c. impedance, UV/VIS analysis of the membranes was also studied.
Keywords
Ion-selective membrane, Sensor, Polymer plasticizers, Ionophores.
1. Introduction
Ion-selective membranes based on ionophore polymeric matrix a now commonly used in a variety of analysis [1-4]. Potassium ions are of special interest of this field owing to their clinical importance.
The aim of this work was to obtain and characterise K+-selective membranes with PVC matrix plasticizer with TCP, doped with crown ethers.
Quantitative electrochemical characteristics such as slope, linear range, selectivity coefficients, were used for the description of membrane functions. The other futures of the membranes, by a.c. impedance measurements, scanning electron microscopy (SEM) and spectroscopy analysis were determinate.
2. Experimental
Reagents and apparatus
All chemicals and solutions were of analytical reagent grade (Merck, Fluka, Reactivul România). Double distilled water was used for the preparation of the solutions. Standard solution (10-1 - 10-5 mol·l-1) with constant ionic strength j = 0,1 (strength adjuster CaCl2 0.033 mol·l-1) were freshly prepared by successive dilution.
For preparation of the K+ - selective membrane, crown ethers (CWE) such as decyl-18-crown-6 (D), dibenso-18-crown-6 (DB), polyvinil chloride (PVC, H.M.W.-powder), tricresylphosphate (TCP), potassium-tetrachis-p-chlorophenyl borate (KTpClPh4B) and tetrahydrofurane (THF) were used.
The apparatus used for the measurement of the physical and electrochemical properties of the membranes were a digital ion analyser (Melter Toledo 355), oscilloscope with double spote (Type 5804, 40MHz), wersatester (E0502), and spectrophotometer (ATI-UNICAM-UV/VIS).
Preparation of K+- selective membranes
For K+ -selective membranes the ionophores (D and DB), TCP as plasticizer KTpClPh4B as lipophilic agent and THF were used [5]. The optimum composition (Wt%) of membranes were 0.5 D or DB; 24.95 PVC; 74.5TCP and 0.25 KTpClPh4B.
3. Results and Discussion
Electrochemical study
A circular section from the master membrane was cut and the electrochemical study of the K+ -selective membrane were made by the following measurement cells:
SCE / internal solution / K+ - selective membrane /
/ K+-sample solution / SCE (double CaCl2 0.33M junction)
Calibration curves (figure 1), and the main electrochemical characteristics (table 1), were obtained.
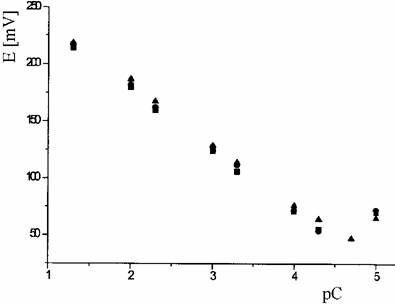
Figure 1. The calibration curves for K+- selective membrane (D):
■ - after conditioning; ● - after 10 days; ▲ - after 20 days
A slight decrease of the slope in time was observed (57 mV/pK to 49 mV/pK), over 6 weeks. The selectivity coefficients, KK,Mpot were evaluated by separate solution method (SSM). The both K+ -selective membranes (D and DB) have a good selectivity for ions of analytical interest.
Impedance measurements
All a.c. impedance measurements of the electrochemical cell were carried out at room temperature, in the range of the frequencies (10-105 Hz). The soaking solution in the cell was 10-2 mol·l-1 KCl. The data was modelling with a Turbo Pascal program [6].
Table 1. Electrochemical characteristics of the K+-selective membrane
|
Electrochemical characteristics |
K+-selective membranes |
|
|
DB |
D |
|
|
Linearity range mol·l-1 |
10-1 - 5·10-5 |
10-1 - 2·10-5 |
|
Calibration slope mV/pK |
53.5 ±3 |
54.5 ±3 |
|
Standard deviation of slope |
1.47 |
1.27 |
|
Correlation coefficient (r) |
0.998 |
0.999 |
|
Detection limit, mol·l-1 |
10-5 |
10-5 |
|
Response time, s |
< 30 |
< 30 |
|
pH range |
2 - 11 |
2 11 |
|
Selectivity coefficient KK,Na KK,NH4 KK,Cu KK,Pb |
6.2·10-2 2.3·10-2 1.5·10-2 4.5·10-2 |
1.7·10-2 8.5·10-3 7.5·10-3 3.5·10-3 |
The impedance spectra, Nyquist plots (figure 2) [7-9] are consistent with an equivalent circuit with the bulk resistance of the membrane, Rh, in series with a parallel Rct/Cdl circuit (Rct is the charge transfer resistance and Cdl is the double-layer capacitance).
The bulk semicircle from the Nyquist diagram is followed by a linear section (Warburg impedance contribution).
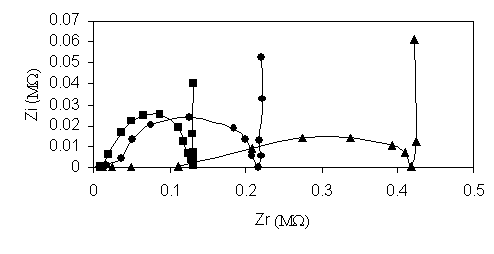
Figure 2. The Nyquist plots of the K+- selective membrane (D)
with different plasticizers:
a) ● - tricresylphosphate; b) ■ - dibutylphtalate; c) ▲ dioctylsebacate
Surface characterisation
For characterising and investigating the structure and surface morphology of the K+ -selective membranes, SEM was used (figure 3).
It shows the initial surface morphology of the K+ -selective membrane. There is a difference between the top surface with no contact with any solid surfaces during casting and bottom surface that was formed in contact with the glass side. The membranes have a no homogeny, uniformity surface, and are transparent.
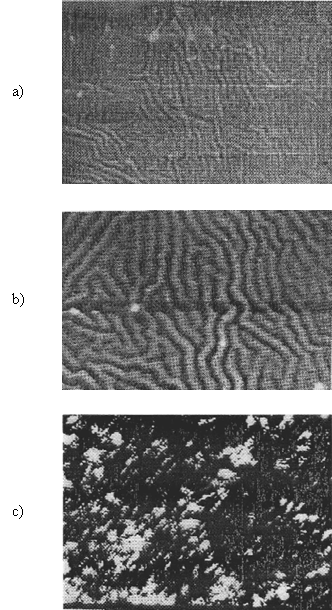 |
Figure 3. SEM images of the K+- selective membrane based on
decyl-18-crown-6 (magnification: ´500);
a) After preparation;
b) After conditioning 24 h in 10-2 mol·l-1 K+;
c) Soaking for a week in 10-2 mol·l-1 K+
After 24 h of conditioning in 10-2 mol·l-1 KCl the surface morphology is shown in figure 3b. Conditioning (to reach electrochemical equilibrium), un-uniformity surface of the membrane, in the same time with of gomflation with loss the transparency, increased.
Figure 3c shown SEM images of the surface of the K+ - selective membrane, after soaking it for a longer period (a week) in 10-2 mol·l-1 KCl. A big change was observed in the surface morphology with the appearance of a sponger aspect. The K+ -selective membrane have a good electrochemical response.
SEM images for both types of membranes (with different ionophors, the same plasticizers), indicate the fact that the surface of the membranes in different stages of functioning suffers modifications, which are consistent with the determined electrochemical, physical (resistance, impedance) parameters.
The surface modifications are the effect of some compositional, technological factors and of the participation in the equilibrium of the K+ -selective membrane.
UV study of the membranes
The transmission UV spectra on the film type membrane have been obtained and are presented in figure 4 and 5.
The spectra of the components through methanol solutions as well as those of the PVC + TCP and PVC + TCP + KTpClPh4B mixtures (slim films), have been obtained and are given in table 2.
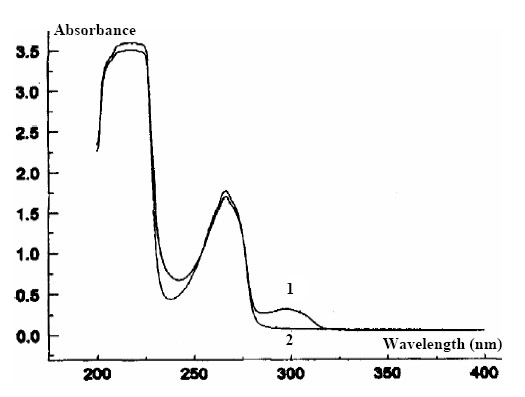 |
Figure 4. The transmission UV spectra of the K+- selective membrane
1) unconditioned; 2) after conditioning
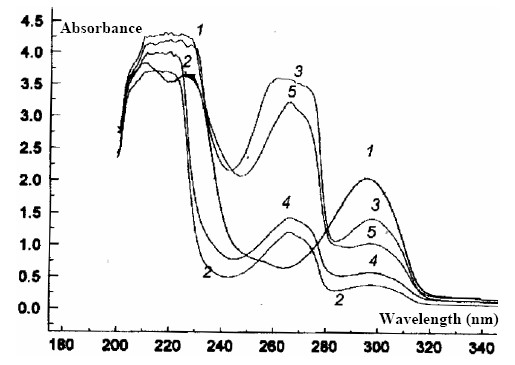
Figure 5. The transmision UV spectra of the K+- selective membrane (s.m.)
1)PVC; 2)PVC+TCP; 3)PVC+TCP+KTpPh4B; 4)K+- s m. (3); 5)K+-s.m. (5)
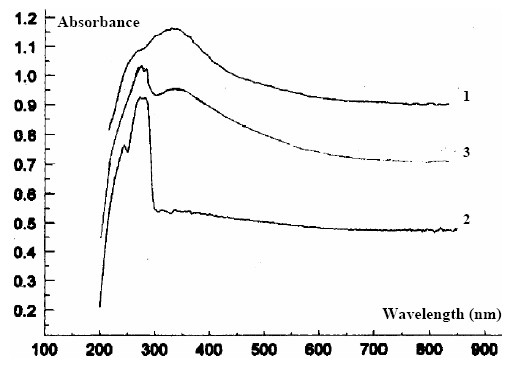
|
Figure 6. The reflection UV spectra of the mother K+-selective membrane
1) unconditioned membrane (one of the faces);
2) conditioned membrane In KCl (same face);
3) conditioned and washed membrane (same face).
In figure 6 is depicted a reflection UV spectra of the mother membrane and was make at different functioning stages.
The transmission UV spectra, both in the case of a DB film membranes and in those of D show a slight displacement of the maximum from l = 280 nm (pure and mixed compounds) to over l = 300 nm, by participation of the crown ethers in the composition of the membranes.
The absorption maximum (l = 300 nm) disappear through conditioning in the 10-2 mol·l-1 KCl solution.
The reflection UV spectra on the dry state (unconditioned) of the mother membrane in are practically similar on the two faces of the membrane.
Table 2. Absorbencies of the pure components in methanolyc solutions
|
Component |
Wave length, [nm] |
Absorbance, A |
|
PVC |
210 230 296 |
3.55 3.52 2.01 |
|
Dibenzo-18-cw-6 |
205 225 274 279 |
1.532 0.978 0.349 0.310 |
|
Tricrezylphosphate |
218 265 269 |
2.485 0.355 0.310 |
|
KTpClPh4B |
210 232 272 281 |
2.471 1.381 0.150 0.100 |
|
Decil-18-cw-6 |
210 230 |
0.300 0.325 |
After conditioning, the reflection UV spectra on the two faces of the membrane from each other and differ from the unconditioned membrane (the maximum of l = 300 nm disappears).
The reflection UV spectrum obtained after several days of soaking in the 10-2 mol·l-1 KCl solution is identical to the spectrum obtained in the case of conditioning for 24 hours.
After washing the membrane, the reflection UV spectrum obtained is similar to the unconditioned membrane (figure 6). The l = 300 nm maximum appears again.
The reflection UV spectra obtained on the membranes with increasing concentrations of crown ether show a displacement of the maximum of l = 300 nm to the right, at the same time with increases of the ether concentration [10].
The modifications appearing in the UV spectrum of the unconditioned membranes with respect to the UV spectra of the pure components can be attributed to the possible interactions within the membranes.
The alterations appearing in the UV spectra of the conditioned membranes (the l = 300 nm maximum disappears) as compared to the unconditioned ones can be caused both by the water interaction (absorption) and to the supplementary interactions of the K+ ion, the ion pair being formed: [crown_ether - K]+[TpClPh4B]-.
4. Conclusions
The physical and electrochemical studies on the K+-selective membranes obtained by crown ethers D and DB was done.
The study shows the fact that the compositional factors are in strict accordance with the physical and electrochemical properties of the K+-selective membranes.
It result that the electrochemical parameters are directly correlated with the physical and optical properties of the membranes in different phases of their functioning.
Experimental determination of the impedance, surface investigation and characterisation and the UV study of the K+-selective membrane are in accordance with electrochemical parameters of these.
References
[1] M. A. Arnold and M. E. Meyerhoff, Crit. Rev. Anal. Chem., 20 (1988), 149.
[2] U. Oesch, D. Ammann and W. Simon, Clin. Chem., 32 (1986), 1448.
[3] J. D. R. Thomas, Anal. Chim. Acta, 180 (1996), 289.
[4] K. Covington and E. Totu, Analyst, 121 (1996), 1811.
[5] E. Hopârtean, E. M. Pică, A. Coroian, V. Cosma and I. Hopârtean, Chemiczna Analyticzna, 46 (2001), 41.
[6] K. Jensen, N. Wirth, Pascal - User Manual and Report, London, 1971, in Matcad 7, Professional 1986, 1997, Math Soft, Inc. U.S. Pat. Nos. 5,469,538 and 5,526,475.
[7] V. V. Coşofreţ, R. P. Buck and M. Erdosy, Anal. Chem., 66 (1994), 3592.
[8] A. Michalska and A. Hulaniczi, Analyst, 119 (1994), 2417.
[9] H. Hara, Y. Kondoh and O. Mitani, Anal. Chem., 62 (1990), 1139.
[10] C. J. Pedersen, J. Am. Chem. Soc., 89 (1967), 126.