Determination of Ni2+ Impurities Using Cadmium-Selective Electrode
Viorica COSMA
C.S. “Senzorom” A.S., Cluj-Napoca, RO, vioricacosma@yahoo.com
Abstract
Taking into account the use of Nickel brasses in industry a great number of methods for the determination of Ni2+ have been reported. More, theoretical predictions of free Cd2+ increasing with decreasing salinity have been confirmed using cadmium selective electrodes.
The method under proposal, for the determination of nickel ions in brass alloys consists of the following steps: Cu2+ masking with sodium thiosulfate; Ni2+ precipitation as nickel-dimethylglyoximate at pH = 5.6 with subsequent selective extraction in chloroform; treatment of the organic phase with HNO3 1M; determination of Ni2+ by titration of the excess of EDTA at pH = 5.6 with a solution of Cd2+, in the presence of the cadmium-selective electrode.
The experimental values showed low dispersion and therefore a good precision. The mean value of nickel content from 13 parallel aliquots of the brass sample was 1.79% which agreed well with those obtained by the atomic absorption spectrometry, 1.77%.
Keywords
Potentiometric determinations; Nickel in brasses; Cd2+-selective electrode
Introduction
Theoretical models are now available that describe response curves in the presence of ion fluxes.[1]-[3] Originally, the relevant ionic concentration profiles were calculated on the basis of fundamental ion-exchange and co-extraction constants.1-3
Recently, a more direct approach has become available that is based on experimentally accessible selectivity coefficients.[4]
Industrial Ni2+ is encountered in the proximity of other elements as a macro component or a micro component. Ni2+ is to be found in arsenates, silicates, sulfides and phosphates together with iron, cobalt, copper, chrome and zinc. Ni2+ is used in industry as such or under the form of alloys like: Monel (a trademark used for an alloy of nickel, copper, iron, and manganese); germane silver (Cu, Zn, Ni); Nichrome (Ni, Cr) and Special Brass (Ni, Cu, Zn).
Actually, choice has been made of the adequate separation and determination techniques by taking into account the Ni2+ concentration, the number and type of accompanying elements as well as the accuracy of the determination.
As a result, the separation can be carried out by selective precipitation, selective extraction separation on ion - exchangers or electrolytic deposition.
A great variety of methods for determination of Ni2+ have been reported.[5]-[9]
From the standard methods in operation, it follows that Ni2+ in brass alloy is determined either colorimetric or photo colorimetric after a previous separation of Cu2+ and Pb2+ by electrolysis.
Method for Ni2+ determination by potentiometric titrations, which make use of different metallic electrodes and different titrant, are also reported.[10]
The elaboration of a method for potentiometric determination of Ni2+ in brass alloys, involving the selective extraction of Ni2+ as dimethylglyoximate, followed by the latter’s determination by the potentiometric titration of the EDTA excess with cadmium nitrate in the presence of the indicator electrode Cd–ISE (Cd2+ ion-selective) has been reported in the present paper.
Experimental procedures
Dimethylglyoxime p.a., ethyl alcohol p.a., copper nitrate p.a., chloroform, cadmium nitrate p.a., complexon III (EDTA), glacial acetic acid, sodium acetate p.a., sodium thiosulphate p.a. and nitric acid p.a reagents was used. A set of solutions was prepared in order to make the experiment: solution of sodium thiosulphate 540 g/l, alcoholic solution of dimethylglyoxime (DMG) 1%, solution of nitric acid 1M, buffer acetic solution with pH = 5.6 (95 ml solution of acetic acid 0.2M + 905 ml solution with 0.2 M of sodium acetate), solution of complexon III 5∙10-3M (T = 0.001881 g/ml), solution of cadmium nitrate 5∙10-3M (T = 0.0002004 g/ml Cd2+) and a synthetic solution estimating the composition of brass (65% Cu, 30% Zn, 2-3% Ni).
The determination have been carried out on a digital pH-meter (1mV accuracy), at room temperature, under stirring (magnetic stirrer). The measuring cell was a Cd-ISE electrode[11],[12] and a double junction saturated calomel electrode (SCE). The glass-electrode has been used in pH - measuring. The Cd-ISE is a solid state electrode with a selective membrane (Ag2S - CdS) and solid electric internal contact (figure 1). The linear range of the electrode response 10-2 - 10-5 M Cd2+ with mV/pCd = 29 ± 3.
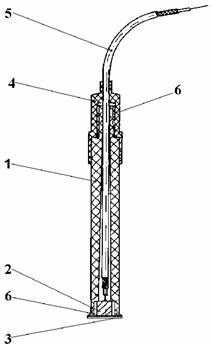
Fig. 1. Cd-ISE
where 1 is body of the electrode, 2 is solid electric internal contact, 3 is selective membrane: Ag2S – CdS, 4 is cover of the electrode, 5 is coaxial cable and 6 is epoxy resin.
Principle of the method
As it has been already said above, Ni2+ is encountered in brass alloys with Cu2+ and Pb2+. In great amounts, Cu2+ precipitates with dimethylglyoxime thus forming a red-brownish precipitate that makes the selective extraction more difficult. That’s why it becomes apparent the necessity of masking Cu2+ with sodium thiosulphate at pH = 5.6, prior to the Ni2+ precipitation with dimethylglyoxime.
The method under proposal, for the potentiometric determination of Ni2+ in brass alloys, where Ni2+ is to by found as a minor component to Cu2+, Zn2+ and Pb2+, consists of the following steps: Cu2+ mashing with sodium thiosulphate at pH = 5.6; Ni2+ precipitation as nickel – dimethylglyoximate at pH = 5.6 and latter’s selective extraction in chloroform; the treatment of the organic phase with 1M nitric acid for the Ni decomplexation and the shifting of it into the aqueous phase; Ni2+ determination by potentiometric titration of the excess of EDTA with a titrated solution of Cd2+, at pH = 5.6, in the presence of the pair of electrodes Cd-ISE and SCE.
Working Mode
The method has been checked out on samples with a well-known amount of Ni2+ that reflects the brass composition and on brass samples (special wire), as well.
The brass (special wire), approximately 4 g is disintegrated with about 50 ml HNO3 1:1 and some drops of concentrated HCl and heated to wet dryness. This is taken again with distilled water and is removed into a graduated flask of 250 ml. Into aliquot of 50 ml of analyzing solutions are added drop wise: 5 ml saturated solution of sodium acetate and 5 ml acetic buffer pH = 5.6 and pH is verified (pH electrode); 5 ml solution of sodium thiosulphate (540 g/l) are added to mask Cu2+ and stirred (the solution changes its color from pale blue to deep blue and after adding thiosulphate it becomes colorless.
The solution thus obtained is introduced into separation funnel, then added 10 ml alcoholic solution of dimethylglyoxime 1% stirred until a red - purplish precipitate is formed; 20 ml CHCl3 are added, then the precipitate is stirred for 2-3 minutes until it solves in the organic phase (inferior layer). The phases are separated and the aqueous phase is submitted to the more extractions with 10 ml and 5 ml of CHCl3, respectively. The organic phases are collected in a separation funnel and washed up with 10 ml distilled water. The organic phase is treated with 10 ml HNO3 1M and is vigorously stirred while the non complex Ni2+ shifting into the aqueous phase. The Ni2+ sample obtained in this way is transferred into a graduated flask of 50 ml. Aliquots of 10 ml each are taken, 5 ml acetic buffer of pH = 5.6 and 15 ml solution of EDTA 5·10-3M are subsequently added.
The complexon excess is titrated with cadmium standard solution and the equivalence point is indicated potentiometrically (Cd-ISE electrode + SCE reference electrode).
Data Analysis and Experimental results
The titration curves are well shaped and show a sharp potential jump at the equivalence (100 mV). The equivalence volumes are calculated by the Hahn-Weiller formula [13].
The amount of Ni2+ is calculated according to the formula:
g Ni2+/sample = MNi · [ VEDTA ·TEDTA / MEDTA - Ve · TCd /MCd] (1)
where MNi, MEDTA and MCd are the respective molecular masses, VEDTA volume of complexon III (EDTA), TEDTA the titer of the complexon III solution and TCd the titer of Cd solution.
Determinations have been made on 13 parallel samples (Table 1) and 3 - 4 titrations have been performed for each sample.
The data obtained have been statistically worked - out in order to establish the precision and accuracy of the method.
The mean value of Ni2+ content in the sample is:
![]() = 6.21· 10-3g Ni2+/sample (2)
= 6.21· 10-3g Ni2+/sample (2)
By means of the relations:
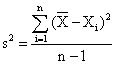
![]() , or
, or 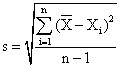
![]() (3)
(3)
the selection dispersion, s2 = 0.64∙10-9 and the standard deviation of the one determination, s = 2.5·10-5 have been calculated.
The product t·s = 5.6 · 10-5 for t95%; k=12 = 2.23, RSD (relative standard deviation) is 0.40%. The confidence range, statistically determined by the formula:
![]() - t·s < X <
- t·s < X < ![]() + t·s (4)
+ t·s (4)
after the replacing of values is: 0.00615 < X < 0.00626.
Table 1. Ni2+ - determination in brass sample (special wire)
|
sample |
g Ni2+/sample |
|
1 |
8.086∙10-2 |
|
2 |
8.115∙10-2 |
|
3 |
8.086∙10-2 |
|
4 |
8.086∙10-2 |
|
5 |
8.411∙10-2 |
|
6 |
8.457∙10-2 |
|
7 |
8.457∙10-2 |
|
8 |
8.262∙10-2 |
|
9 |
8.260∙10-2 |
|
10 |
8.260∙10-2 |
|
11 |
8.115∙10-2 |
|
12 |
8.086∙10-2 |
|
13 |
8.086∙10-2 |
The values obtained are included in the confidence range, which means that the method is accurate. A very restricted confidence range is noticed about the average value (t∙s = 5.6∙10-5) as well as a very good proximity to the mean value of the 13 samples versus the real value, as such we may conceive that the method proves precision and accuracy.
A
brass sample was analyzed using the method above described. Determination was
been made on parallels aliquots of the brass sample and the results have been
compared with those obtained by the atomic absorption spectrometry (A.A.S). The
experimental values show low dispersion and therefore a good precision. The
calculated mean value of nickel content ![]() = 1.79 % the selection dispersion s2
= 1.083∙10-3, s = 3.29∙10-2, t∙s = 7.17∙10-2
(t95%, k=12 = 2.18) and RSD = 0.18%.
= 1.79 % the selection dispersion s2
= 1.083∙10-3, s = 3.29∙10-2, t∙s = 7.17∙10-2
(t95%, k=12 = 2.18) and RSD = 0.18%.
The mean value obtained by the atomic absorption spectrometry (A.A.S.) is 1.77% Ni2+.
The comparison of the mean value obtained by the method above described with that obtained by A.A.S. using the “t” criterion, lead us to the conclusion that our method is accurate; tcalculated = 2.16; t95%; k =12 = 2.18.
Conclusions
The proposed method is based on the selective extraction of Ni2+ - dimethylglioximate followed by its determination with the help of the potentiometric titration of EDTA excess with cadmium nitrate in the presence of Cd-ISE. This method has a good precision and accuracy and can be efficiently used.
References