Molecular Descriptors Family on Structure Activity Relationships
3. Antituberculotic Activity of some Polyhydroxyxanthones
Sorana BOLBOACĂ, Lorentz JÄNTSCHI
“Iuliu Haţieganu” University of Medicine and Pharmacy, http://sorana.academicdirect.ro
Technical University of Cluj-Napoca, Romania, http://lori.academicdirect.org
Abstract
The antituberculotic activity of some polyhydroxyxanthones was estimated using the Molecular Descriptors Family on Structure Activity Relationships methodology. From a total number of 298110 real and distinct calculated descriptors, 94843 were significantly different and entered into multiple linear regression analysis. The best performing bi-varied model was obtained by use of all polyhydroxyxanthones. The MDF SAR model was validated splitting the molecules into training and test sets. A correlated correlations analysis was applied in other to compare the MDF SAR models with the previous SAR model. The prediction ability of antituberculotic activity of polyhydroxyxanthones with MDF SAR methodology is sustained by three arguments: leave-one-out procedure, training vs. test procedure, and the correlated correlations analysis. Looking at the bi-varied MDF SAR model, we can conclude that the antituberculotic activity of polyhydroxyxanthones is almost of geometrical nature (99%) and is strongly dependent on partial atomic charge and group electronegativity.
Keywords
Molecular Descriptors Family on Structure Activity Relationships, Polyhydroxyxanthones, Antibuberculotic activity
Introduction
Xanthones comprise a group of compounds found in dill, gentian, and henna. Polyhydroxyxantones are known to have antituberculotic activities against Mycobacteriun Tuberculosis [[1]], and had been highlight in the spectroscopic researches [[2],[3]].
A previous study analyzed the antituberculotic activity of polyhydroxyxantones [[4]] using the partial least squares method and a structure-activity relationship was reported:
· number of compounds: 10;
· number of components (dependent variables): 4;
· squared correlation coefficient, r2 = 0.986;
· cross-validated r2, 0.613.
The aim of the research was to study the ability of MDF SAR modeling in prediction of the antituberculotic activity against Mycobacteriun Tuberculosis of polyhydroxyxanthones.
Material and method
A number of ten polyhydroxyxanthones with antituberculotic activity were included into the study [4]. The polyhydroxyxanthones, the measured and the previous reported antituberculotic activity are in table 1.
The antituberculotic activity, expressed in MIC (minimum inhibitory concentration) units [μmol/mL], is the lowest concentration of the polyhydroxyxanthones required to inhibit the growth of Mycobacterium Tuberculosis in vitro.
The steps of molecular descriptor family on structure activity relationship (MDF SAD) modeling of antituberculotic activities of polyhydroxyxanthone compounds are [[5]]:
· Step 1: Sketch of the polyhydroxyxanthone compounds;
· Step 2: Create the polyhydroxyxanthone compounds antituberculotic activities file;
· Step 3: Generate the polyhydroxyxanthone compounds MDF members;
· Step 4: Find the antituberculotic activity SAR models;
· Step 5: Validate and compare with previous reported results the MDF SAR models;
· Step 6: Analyze the selected MDF SAR model.
Table 1. The polyhydroxyxanthones, measured and previous calculated
anti-tuberculotic activities (AntiTb)
|
Polihydroxyxanthone Structure |
Measured AntiTb |
Calculated AntiTb, from [4] |
|
|
x01 |
1.33 |
1.42 |
|
|
x02 |
|
1.33 |
1.29 |
|
x03 |
|
1.33 |
1.30 |
|
x04 |
|
2.00 |
1.99 |
|
x05 |
|
1.33 |
1.29 |
|
x06 |
|
1.00 |
1.03 |
|
x07 |
|
0.33 |
0.43 |
|
x08 |
|
0.67 |
0.69 |
|
x09 |
|
0.33 |
0.24 |
|
x10 |
|
1.33 |
1.30 |
Results
In modeling of antituberculotic activity of polyhydroxyxanthones using MDF SAR methodology, from the total possible number of molecular descriptor members (787968), 298110 had real and distinct values. Using a 10-9 significance selector to bias the values, the MDF members had been reduced to a number of 94843 significantly different molecular descriptors. In order to obtain a bi-varied MDF SAR model, pairs of descriptors was correlated with measured antituberculotic activity of polyhydroxyxanthones, by use of a MLR (Multiple Linear Regression) procedure. The best performing pair of descriptors is (lHPDOQg, IsMRKGg). The calculated values of molecular descriptors and the estimated values for antituberculotic activity are in table 2.
The bi-varied MDF SAR model (Estimated AntiTb, Ŷ from table 2) has the following associated statistics:
r = 0.999; r2 = 0.997; r2adj = 0.997; r2cv = 0.995; F = 1330; pF = 9·10-8 %;
r2(AntiTb, lHPDOQg) = 0.48; r2(AntiTb, IsMRKGg) = 0.71
Table 2. The calculated values of the molecular descriptors and estimated by MDF SAR antituberculotic activities
|
Molecule |
lHPDOQg |
IsMRKGg |
Estimated AntiTb, Ŷ |
|
x01 |
-1.04410 |
1.18240 |
1.34 |
|
x02 |
-0.65994 |
1.13610 |
1.33 |
|
x03 |
-0.94910 |
1.16870 |
1.29 |
|
x04 |
-0.59172 |
1.16090 |
1.97 |
|
x05 |
-0.58128 |
1.12600 |
1.32 |
|
x06 |
-0.57953 |
1.11070 |
1.03 |
|
x07 |
-0.48518 |
1.06220 |
0.31 |
|
x08 |
-0.48318 |
1.08020 |
0.66 |
|
x09 |
-0.74112 |
1.09400 |
0.33 |
|
x10 |
-0.69026 |
1.14230 |
1.38 |
Ŷ = -19 + 2.3·lHPDOQg + 19·IsMRKGg
The plot of the bi-varied MDF SAR model from table 2 is in figure 1. The external validation of the best bi-varied MDF SAR model was appraised using the training vs. test experiment. The molecules were successively split into training and test sets. For every training set sample size from four to seven, two random selections were made. Table 3 contains the molecules entered in training sets, the MLR results (coefficients, the squared correlation coefficient) for training set, and the squared correlation coefficient values for test sets (which contain the rest of molecules).
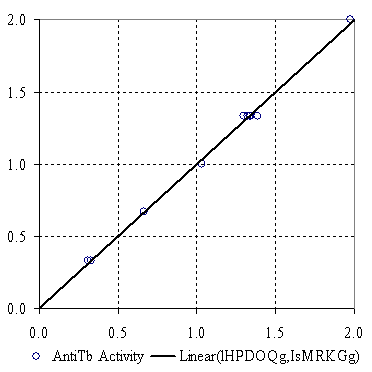
Figure 1. Measured vs. bi-varied MDF SAR calculated antituberculotic activity values
Table 3. Training vs. test sets results using bi-varied MDF SAR model
|
Training set |
Test set |
||||
|
Molecules |
Intercept |
lHPDOQg |
IsMRKGg |
r2 |
r2 |
|
8, 7, 9, 6 |
-17.93 |
2.28 |
18.23 |
0.999 |
0.980 |
|
1, 8, 5, 9 |
-19.18 |
2.37 |
19.43 |
1.000 |
0.995 |
|
10, 4, 7, 9, 3 |
-19.13 |
2.30 |
19.35 |
0.997 |
0.997 |
|
10, 8, 6, 3, 5 |
-17.80 |
2.02 |
18.00 |
0.991 |
0.996 |
|
10, 5, 8, 6, 9, 1 |
-18.64 |
2.26 |
18.88 |
0.997 |
0.999 |
|
4, 2, 6, 3, 7, 1 |
-19.24 |
2.33 |
19.47 |
0.998 |
0.997 |
|
7, 3, 2, 9, 10, 1, 8 |
-18.50 |
2.20 |
18.72 |
0.997 |
0.999 |
|
7, 3, 2, 9, 8, 4, 10 |
-19.09 |
2.30 |
19.32 |
0.998 |
0.995 |
The correlated correlations analysis between the bi-varied MDF SAR model and the previous reported SAR (PrevRep) was performed using the Steiger’s Z test. The results are:
r(AntiTb, MDF SAR) = 0.999; r(AntiTb, PrevRep) = 0.993; r(MDF SAR, PrevRep) = 0.992;
Z = 2.138; pZ = 1.63%
Discussions
The bi-varied model uses the lHPDOQg and IsMRKGg MDF members, which both consider the geometrical shape of the molecules (g) as distance metric operator. As atomic property, one of descriptor takes into consideration the partial charge (Q) and other one the group electronegativity (G). The probability of wrong model is equal with 9·10-8%. Ninety-nine percent of variation in antituberculotic activity it is explainable by its linear relation with lHPDOQg and IsMRKGg MDF members. The cross-validation leave-one-out correlation score of bi-varied MDF SAR model demonstrate the power of the model in antituberculotic activity of polyhydroxyxanthones prediction (r2cv = 0. 995).
The external validation of the bi-varied MDF SAR model and its ability in prediction of the antituberculotic activity of polyhydroxyxanthones is demonstrated by the results of training vs. test experiment (table 3). The averages of squared correlation coefficients from training (0.997) and test (0.995) are similarly to the model squared correlation coefficient (0.997) and cross-validation leave-one-out squared correlation coefficient (0.995), which prove its ability in prediction. The bi-varied MDF SAR model has significantly better ability in prediction of antituberculotic activity of polyhydroxyxanthones compared with the previous reported SAR model (see pZ = 1.63% from Steiger’s Z test).
Conclusions
The bi-varied MDF SAR mode has better ability in prediction of antituberculotic activity of polyhydroxyxanthones compared with the previous reported SAR model.
The antituberculotic activity of the polyhydroxyxanthones is almost of geometrical nature (99%), and is strongly dependent on partial atomic charge and group electronegativity.
References