Production of Dextrins from Cassava Starch
O. S. AZEEZ
Chemical Engineering Department, Federal University of Technology, Minna,
Niger State, Nigeria, azeezsarafa2002@yahoo.com
Abstract
This research work is aimed at producing dextrins from cassava starch and also to investigate the effects of acid concentration, roasting temperature and time on dextrin produced using 8 run screen experimental design before the preparation of adhesive.
It was shown in the result that the best of white dextrin, yellow dextrin and British gum were obtained at concentrations of acid of 0.3M, 0.3M, and 0.23M and at roasting time of 30, 180 and 180 minutes and at roasting temperature of 60oC, 90oC and 120oC respectively.
The best of white dextrin which had the highest solubility of 36.1 per cent was used to formulate the adhesive.
Keywords
Dextrin, Cassava starch, White dextrin, Yellow dextrin, British gum
Introduction
Adhesives are polymer products, which are capable of joining two materials together by surface attachment, or any material having the properties of adhering. This property of adhering is however, a developed property of the substance. The attachment of surfaces is called adhesion, i.e. a solid surface and a second phase which could either be made up of individual particles, molecules of liquid or solid of continuous film. Processing of adhesives depends mainly on rheological parameters and the quality of the final product is determined by the interaction between adhesive and adherand.
Lagrange (1804) made dextrins by roasting starch; he was attempting to find a substitute for gum Arabic, which was then largely used in industries.
British gum was however a coincidental discovery which originated as a result of fire in a starch storage building at a textile plant near Dublin Ireland in September 1921. Part of the factory was destroyed by fire and the stored starch, which had been exposed to heat roasted to a brownish yellow colour. When this was mixed in cold water it no longer had the characteristic of a starch but dissolved to a viscous gummy liquid. The result was duplicated by heating starch in a working pot over fire and its result was henceforth known to be British gum.
Dextrins was made by moistening dried starch with dilute mixture of acid (HCl) and nitric acids (HNO3), the mixture was spread in a corrugated iron sheets and exposed to the heat of a bakers oven until they were thoroughly dried and slightly yellow.
Dextrins exist in various forms but their characteristics and mechanical properties depend on the details of manufacture i.e. time of roasting, use or non-use of converting agent, character of wavering agents, raw materials, concentration of acid used or mixtures of different starches.
The colours of dextrins ranges from a pure white to a dark brown, some grades are highly adhesive and dry quickly. Others are different in viscosity and show a lower degree of adhesion and less drying rate.
The major raw materials for dextrins are the starches such as corn, potato, tapioca (cassava) with smaller uses of starches from other sources. However, for this research work cassava roots also known as tapioca is the source of starch used.
Design of the experiment
The experimental design table that was chosen was used to ensure proper distribution of the process variables to arrive at a reasonable conclusion for the optimum conditions for the production of starch with respect to the chosen variables i.e. concentration of acid, roasting time and roasting temperatures the eight run screening design algorithm was used.
The extremes of the ranges of the variables represent the low and high factor levels. The eight runs are useful for the estimation of main effects and interactions. The response error could be estimated by adding a third level of each factor which is known as (cp). The centre point of each factor is located at the centriod of the factor range. The table is formulated as shown below.
Table 1. 8-Run Screening Design
|
Run |
X1 |
X2 |
X3 |
|
1 |
- |
- |
- |
|
2 |
+ |
- |
- |
|
3 |
- |
+ |
- |
|
4 |
+ |
+ |
- |
|
5 |
- |
- |
+ |
|
6 |
+ |
- |
+ |
|
7 |
- |
+ |
+ |
|
8 |
+ |
+ |
+ |
|
9cp |
0 |
0 |
0 |
|
10cp |
0 |
0 |
0 |
|
11cp |
0 |
0 |
0 |
Experimental Work
Fifty grams of dried cassava starch was weighed into a beaker and then sprayed with 0.3M HCl and vigorously stirred.
It was allowed to age for 24 hours.
It was then heated at a temperature of 60°C for 60 minutes after which a sticky ash brown solution was formed.
The content was then cooled in a water bath until the temperature of the pasty mass was the same as the room temperature.
This same procedure was repeated for the remaining experiment but the concentration of acid, roasting temperature and roasting time were now varied for the three types of dextrin.
Results
Experimental Design of Dextrinization Process Parameters
Table 2. Dextrinization Process Parameters for White Dextrins
|
Run |
Roasting temperature (°C) |
Conc. of acid used (M) |
Roasting time (min.) |
Relative solubility (%) |
|
1 |
60 |
0.300 |
30 |
36.1 |
|
2 |
80 |
0.300 |
30 |
33.0 |
|
3 |
60 |
0.350 |
30 |
32.0 |
|
4 |
80 |
0.350 |
30 |
32.2 |
|
5 |
60 |
0.300 |
90 |
33.6 |
|
6 |
80 |
0.300 |
90 |
34.2 |
|
7 |
60 |
0.350 |
90 |
33.8 |
|
8 |
80 |
0.350 |
90 |
33.5 |
|
9 |
70 |
0.325 |
60 |
31.0 |
|
10 |
70 |
0.325 |
60 |
30.0 |
|
11 |
70 |
0.325 |
60 |
28.2 |
Estimate of response error using replicate:
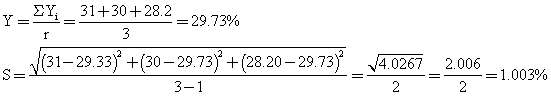
with (r-1) = 3-1 = 2 degree of freedom.
Table 3. Dextrinization Process Parameters for Yellow Dextrins
|
Run |
Roasting temperature (°C) |
Conc. of acid used (M) |
Roasting time (min.) |
Relative solubility (%) |
|
1 |
70 |
0.250 |
60 |
12.00 |
|
2 |
90 |
0.250 |
60 |
18.00 |
|
3 |
70 |
0.300 |
60 |
16.18 |
|
4 |
90 |
0.300 |
60 |
21.80 |
|
5 |
70 |
0.250 |
180 |
18.00 |
|
6 |
90 |
0.250 |
180 |
15.60 |
|
7 |
70 |
0.300 |
180 |
19.30 |
|
8 |
90 |
0.300 |
180 |
32.16 |
|
9 |
80 |
0.275 |
120 |
21.10 |
|
10 |
80 |
0.275 |
120 |
20.00 |
|
11 |
80 |
0.275 |
120 |
18.40 |
![]()
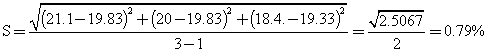
with (r-1) = (3-1) = 2 degree of freedom.
Table 4. Dextrinization Process Parameters for British Gum
|
Run |
Roasting temperature (°C) |
Conc. of acid used (M) |
Roasting time (min.) |
Relative solubility (%) |
|
1 |
100 |
0.230 |
60 |
18.8 |
|
2 |
120 |
0.230 |
60 |
19.2 |
|
3 |
100 |
0.280 |
60 |
18.7 |
|
4 |
120 |
0.280 |
60 |
15.8 |
|
5 |
100 |
0.280 |
180 |
14.9 |
|
6 |
120 |
0.230 |
180 |
19.7 |
|
7 |
100 |
0.280 |
180 |
15.4 |
|
8 |
120 |
0.280 |
180 |
16.4 |
|
9 |
110 |
0.280 |
180 |
15.2 |
|
10 |
110 |
0.255 |
120 |
14.8 |
|
11 |
110 |
0.255 |
120 |
18.6 |

with (r-1) = (3-1) = 2 degree of freedom.
Estimation of Interaction Effects
The possible interaction in this analysis is: C∙T, C∙t, T∙t, C∙T∙t.
Table 5. Interaction Effects
|
Run |
C·T |
C·t |
T·t |
C·T·t |
|
1 |
+ |
+ |
+ |
- |
|
2 |
- |
+ |
- |
+ |
|
3 |
- |
- |
+ |
+ |
|
4 |
+ |
- |
- |
- |
|
5 |
+ |
- |
- |
+ |
|
6 |
- |
- |
+ |
- |
|
7 |
- |
+ |
- |
- |
|
8 |
+ |
+ |
+ |
+ |
|
+ |
92 |
81 |
18,000 |
5,490 |
|
- |
90 |
75 |
15,600 |
5,430 |
|
Difference |
2 |
6 |
2,400 |
60 |
|
Effect |
0.5 |
1.4 |
600 |
15 |
An interaction column is formed multiplying the entities in the column that comprises the interaction, where C = concentration of acid, T = Roasting temperature, t = time. Thus the C·T is the product of C and T columns in table 5.
Since the main effects for C·t, T·t and C·T·t are large it means the interaction of concentration of acid temperature and time of roasting are significant.
Discussion of Results
The process of dextrinization was followed, bright coloured dextrins i.e. (sparkling white) with the best solubility was obtained at a small cost i.e. heating and stirring. The range of temperatures was from 60°C to 120°C. Temperature lower than 60°C was found unsuitable since the desired dextrins were not formed. At higher temperature it was observed that dextrinization started prematurely and with formation of clots, the favourable temperature was found to be in the neighbourhood of 60oC-80oC.Table 2 shows that while dextrins were conveniently prepared with moderate acid concentration of 0.3M-0.35M and at temperature range of 60°C 80°C. At this temperature and at a time interval of 30-90 minutes, the conversion of starch to dextrin was seen to be gradual and very easy to monitor and control. They were however rapid at higher temperatures and of brownish colours. They were found to be very soluble in water with their relative solubilities ranging from 28.2 36.1. From the table it is evident that higher concentrations of acids and at moderate temperature gave the best solubilities i.e. table 2 which gave highest range of solubilities. The solubility of other dextrins was also okay. Since they were soluble to satisfactory extent in water, which correspond to what we have in literature.
When bottle labelling glue was compounded from the dextrin, fine glue was obtained, the stickiness was satisfactory and the glue dried within 30-60 seconds after application. In table 3, yellow dextrin was obtained, they were also soluble in water, British gum was prepared at very high temperature range as shown in table 4, and the conversion was much more rapid than in yellow and white dextrin. The method of experimental design used also shows that response error estimated based on the experimental analysis were as follows for white 1.003%, for yellow 0.791% and for British gum 1.47%, which are bearable.
In the estimation of the main effect, for a factor to be significant, the main effect for that factor should be large, and for all the three types of dextrin produced, concentration of acid, Temperature and time of roasting are all significant and in the interaction effect, the interaction of temperature, time and volume of acid used are significant.
From the table we can see the optimal conditions necessary for the preparation of each dextrin.
Table 6. Optimum Condition of Temperature, Concentration of acid and Roasting time
|
Type |
Roasting temperature (°C) |
Concentration of acid used (M) |
Roasting time (min.) |
|
White |
60 |
0.3 |
30 |
|
Yellow |
90 |
0.3 |
180 |
|
British Gum |
120 |
0.23 |
180 |
The above table shows that from the experiment the best of white dextrins was produced at a concentration of 0.3M and a temperature of 60°C at a time interval of 30 minutes.
That of yellow dextrins was produced at a concentration of 0.3M at a temperature of 90°C and at a time interval of 180 minutes.
British gum was also at its best solubility when a concentration of 0.23M of HCl acid was used at a temperature of 120°C and at a time interval of 180 minutes.
From the results it will be noticed that high concentrations and moderate temperatures give better results and that increased temperatures and time range at lower concentration had less effect.
Conclusions
The best type of white dextrin is obtained when 0.3M of HCl was used to spray starch at a temp of 60°C for 30 minutes.
It can also be deduced that high temperature and prolonged period of heating gives the best of yellow dextrins while that of British gum was achieved at a much higher temperature at a longer time but at low concentration of acid.
All dextrins were relatively soluble in water.
The interaction effect of concentration of acid used, time and temperature of roasting in the preparation of dextrin is very significant.
Recommendation
The action of HCl is too drastic and that of HNO3 being too mild, hence, the two types of acid are mixed for comparison and to obtain better results.
The range of parameters used in this work could be increased or decreased to see the effects on the dextrins formed.
References
[1] Azeez O. S., Preparation of Adhesive from Cassava Starch, B.Sc Thesis, Department of Chemical Engineering, Obafemi Awolowo University, Ile Ife, Osun State, 1994.
[2] Adeboye A., Production of Adhesive from Cassava Starch, B. Sc. Thesis, Department of Chemical Engineering, Federal University of Technology, Minna, Niger State, 2003.
[3] Encyclopaedia Britannica Vol. 1, 15th Edition, 1974.
[4] Monteel C. L., The Water Soluble Gum, Hafner Publishing Company, New York & London, 1947.
[5] Murphy T. D., Design and Analysis of Industrial Experiments, Chemical Engineering, New York, 1977.
[6] Robert L. D. and Marshell S., Adhesive and Adhesive Action, Logmer, Green and Co. Ltd., London, 1968.