A Review of Advances and Quality Assessment of Biofuels
Mohammed UMAR GARBA, Mohammed ALHASSAN, Abdulsalami S. KOVO
Department of Chemical Engineering, P.M.B. 65 Federal University of Technology Minna
tanimugarba@yahoo.com, mohakusi2003@yahoo.com, kovoabdulsalami@yahoo.com
Abstract
The search for the exploitation of renewable and environmental benign energy resources increase globally due to increasing prices and uncertainties in the availability of conventional energy resources such as mineral fuels. Biofuels are alternative renewable fuels that have received considerable attention in the recent past: namely bioethanol, which is made from crop grains or sugarcane and biodiesel, which is made from vegetable oils and animal fats, and both are used as a sources of power for cars, trucks, and aircrafts. Presently the fuels are produced and marketed in Brazil and many developed countries, and serves as a potent supplement/substitute for fossil fuel. This paper examines the precedence of biofuel as a fuel and highlights the advances made in the production, use and quality assessment at the global level.
Keywords
Biofuels, Quality assessment
Introduction
Bio-fuels are alternative renewable energy that has received considerable attention in the recent past, among the well known bio-fuel include namely bio-ethanol, which is made from crop grains or sugarcane and bio-diesel, which is made from vegetable oils and animal fats and both are used as sources of power for cars, trucks and aircraft. German engineer Rudolf Diesel’s first eponymous engine in 1897 was ran using peanut at Paris exposition. Henry Ford one of the pioneers of automobile manufacture designed his equipment to run on ethanol. These visionary inventors expected that their new machines would run on fuels derived from plants, but cheap petroleum proved more popular (Goering et al, 1982). The oil crises of the 1970 rekindled interest in the use of renewable fuels and the following main factors sustained this interest to date.
· Prices of petroleum products have been on the increase since the time of the oil crises.
· Uncertainties in oil supplies due to political instability and conflicts in some oil producing areas of the world
· Growing anxiety over the future security of the world s’ supply of crude because petroleum resources are limited in stock.
Research and development efforts in biofuels to supplement or substitute petroleum fuels focus on these two major. The world’s larger producers are Brazil (ethanol), U.S.A (ethanol), Germany (biodiesel), and Austria (biodiesel) respectively. Most of Brazil’s 20 million drives tank up with a fuel that is cut with 25 percent ethanol. In Europe 1.933x106 tones of biodiesel were produced in 2004 (EBB, 2005). The boom has some powerful institutions behind it. As governments across the globe come to grips with global warning, biofuels are seen as a pragmatic step toward reducing carbon emissions. A glowing number of countries now require various quality assessments to ensure that the renewable fuel complies with approved specification and standards.
The objective of this paper was to review the progress made in the search for exploitation of renewable and environmental benign biefuels and to highlight on the production, advances, use, and quality assessment.893
Factors leading to biofuel production
Early research on renewable liquid fuels focused on the use of raw vegetable oil from field and tree crops as a mineral diesel substitute or supplement to run diesel engines. Many researchers have reported on the use of raw vegetable oil, other wise called pure, neat or straight vegetable oil (SVO) as fuel in both refined and unrefined forms. Peterson et al. (1893), Schlautman et al. (1986) and Yong (1998), M. Alhasan et al (2005) used sunflower, soybean and cotton seed oils to power diesel engines respectively. The oils were blended with petroleum diesel in various volumetric proportions. Also, McDonnel et, al. (1995) used deguimmed rapeseed and sunflower oils blender with petroleum to fuel diesel engines. While Dorado et al. (2002) used a 10% waste vegetable oil and 90% degummed diesel blend.
A blend of degummed rapeseed oil petroleum diesel (especially with high proportions of the) was found to be suitable fuel for conventional diesel enzymes (McDonnal et al, 1995). However, all researchers unanimously agree that the relatively high viscosity as well as low volatility of SVOS (Monyem et al, 2001) relatively compared to petroleum diesel is a major disadvantage in using them as fuels or fuel supplements. High viscosities of the oils reported above resulted in the following enzyme problems, especially after long-term tests:
· Loss of power (6:7-12%) due to poor atomization.
· Increase in enzyme oil viscosity due to deposit resulting from incomplete combustion.
· Coking of injector nozzle tips.
· Abnormal carbon deposit in engine parts.
· Sticking and wearing of pistons, rings and cylinders.
· Excessive smoke emissions.
Definition, composition and method of biofuel production
Bio-fuel is a generic term for transport fuel that can be produced from renewable material of plant or animals origin and are substitutes or partial substitute for fossil, (or mineral) fuels. Bio-ethanol is an alcohol made from sugar, starch and products containing sugars and starches through a process of fermentation and distillation, and used as a substitute or partial substitute for gasoline. Bio-diesel can be produced from any vegetable oil or animal’s fat these fats or oils are chemically converted to esters and used as a substitute or partial substitutes for mineral diesel. Technically, bio-ethanol is defined as ethyl alcohol derived from grain crops or sugar for use in patrol (gasoline) motor while biodiesel is defined as mono alkyl esters derived from vegetable oils fats use in compression (diesel) enzyme. Pure or 100% biofuel is designated as BXX where XX indicates the volumetric percentage biofuel contained in the blend. Thus, a B20 blend 20% and 80% of bio-fuel and mineral by volume respectively. The most common starchy plant used in bio-ethanol production is sugarcane in Brazil, U.S.A and china. However, all starchy plant can be used as feed stock. The biochemical pathway of fermentation in the production of ethanol is known as broken down to pyrulic acid; a 3-carbon compound which is further reduced to from ethyl alcohol), and it is depicted in equation I below (Mathewson, 1980). The starchy grains like corn are ground up and mixed with water. Other grains must first soak up to 48 hours. Enzymes convert the starches into a simple sugar called dextrose. Yeast is added and fermentation begins.
After 40 to 50 hours by products are filtered out and ethanol is distilled again 200 proof before stripping. The following three methods of producing bio-ethanol are common.
C6 H12 O6 → 2C2 H5OH +2CO2 + heat (1)
The starch product (guinea corn cassava) is crushed and heated in steam under pressure to extract the starch. The starch is then converted into sugar with the aid of catalyst.
The cellulose product (cotton Lint) is diluted in acid and heated under pressure, this convert the fiber into glucose sugar.
(i) Molasses is dilutes with water and slightly acidified yeast is added and the mixture fermented for 3 or 4 days.
The most common oil used in biodiesel production is soybean oil in U.S.A and rapeseed oil in Europe. However all vegetable oils animal fats can be used as feed stock. The chemical process involved in the biodiesel production is called esterification.
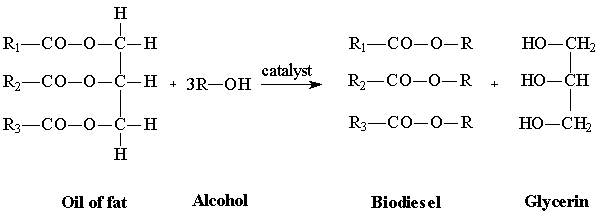
Figure 1. Chemical reaction for bas catalyzed biodiesel production (N.B.B. 2002)
The formation of at least one ester depicted in Figure 1 above from the figure, R is a short chain hydrocarbon in the alcohol; R1, R2, and R3 are fatty acid chains associated with oil or fat, which are largely palmitic, stearic, oleic and lin oleic acids for naturally occurring oils and fats (catalysts 1%).
Depending on the feedstock and alcohol used, a feed quality fat may be
produced in place of the fertilizer component shown above, while the glycerine
formed as a by – product can be used in the cosmetic and pharmaceutical
industries. During the esterification process, the triglyceride in vegetable
oil or animal fat reacts with an acohol (normally methanol or ethanol) in the
presence of catalyst that has already been mixed the alcohol to form alkyl
ester (biodiesel) and glycerine. The alcohol is charged in excess to assist in
quick. Esterification is used as a simple method for adapting plant to the
requirements or specifications of diesel engines and the mass balance of the
process inputs and outputs is as follows (NBB, 2001):
Catalyst (1%)
Veg. oil (87%) +Alcohol (12%) →
Biodiesel (80%) + Alcohol (4%) + Glycerine (9%) + Fertiliser (1%) (2)
Depending on the feedstock and alcohol used, a feed quality fat may produce in place of the fertilizer component shown above (Shumaker et al., 2003). While the glycerine formed as a by-product can be used in the cosmetic and pharmaceutical industries. During the esterification process, the trigliceride in vegetable oil or animal fat reacts with an alcohol (normally methanol or ethanol) in the presence of a catalyst that has already with an alcohol to form mono alkyl esters (biodiesel) and glycerine. The alcohol is charged in excess to assist in quick conversion (NBB, 2002). The fuel produced consists of a maximum of 6 – 7 fatty acids, depending on the feedstock used (Krahl et al., 2003). The following three methods of producing biodiesel are common (NBB, 2002; NBB, 2005):
a. Base catalysed transesterification of oil using an alkaline (base) catalyst such as sodium hydroxide, potassium hydroxide and sodium methoxide;
b. Direct acid catalysed esterification using an acid catalyst such as sulphuric, sulphonic and hydrochloric acids;
c. Conversion of oil to fatty acids and then to alkyl esters through acid catalysis.
The first method is deemed to be most common and most economical (NBB. 2002; Shumaker et al., 2003) and is used by commercial producers because it is fast. The second method is favored for esterification of waste oils such as restaurant oils and frying oils due to their free fatty acid contents, which are not compatible with alkaline catalysts.
A related most method used in the production of the so-called synthetic biodiesel is termed as the biomass-to liquid (BTL) process in which solid feedstock is converted into charcoal and a gas. The gas is purified and liquefied through the Fischer-Tropsch reaction and used as biodiesel (USDA, 2005).
Biodiesel production is considered to be technically simple and a “home-brewing” trial has been reported. A test-batch mini-processor for producing 1-2 considerably reduces the viscosity (the major disadvantage) of vegetable oil from a range of 11 - 17 times (Peterson et al., 1983) to just about twice that of petroleum diesel. Table 1 presented some fuel properties of two B100 biodiesel types (rapeseed methyl ester and rapeseed ethyl ester) and those of high-grade petrodiesel.
Table 1. Some fuel characterization data for biodiesel (B100) and No. 2 diesel (D2)
|
Special properties |
RMEa |
REEb |
D2 |
|
Specific gravity |
0.8802 |
0.876 |
0.8495 |
|
Viscosity @ 40oC |
5.65 |
6.11 |
2.98 |
|
Cloud point (oC) |
0 |
-2 |
-12 |
|
Pour point (oC) |
-15 |
-10 |
-18 |
|
Flash point (oC) |
179 |
170 |
74 |
|
Boiling point (oC) |
347 |
273 |
191 |
|
Cetane number |
61.8 |
59.7 |
49.2 |
|
Sulphur (%wt) |
0.012 |
0.012 |
0.036 |
|
Gross heat of combustion |
40.54 |
40.51 |
45.42 |
|
Net heat of combustion (MJ/kg) |
37.77 |
37.82 |
42.90 |
|
aME- represented methyl ester,bREE – represented ethyl ester Source: Adapted from Peterson and Reece (1996) |
|||
Considerable amount of work has done at laboratory and filed/road levels to assess the performance of biodiesel and its blends in C1 engines.
Recent advances made in biofuels production
One of the very recent advances in biofuel is bio-distillation of the biomass. Bio-distillation convert biomass oil into hydrocarbon fuel using existing petroleum refinery technology with minor modifications. Bio-distillation was ranked number one for several reasons. This benefit of approach are significant in that production and distribution costs can be minimized, the existing infrastructure is used to (no duplicate infrastructure ) and key political barriers are addressed.
Another important advances made in biomass oil production is the reduction in cost using supercritical carbon-dioxide oil extraction technology. It offers some benefit in terms of lower cost, higher oil quality (less pretreatment required) is suitable for smaller plants.
There is an increasing in oil seed improvement. Expanding supplies are necessary to maximize petroleum displacement potential. Improvement can also reduce oil production costs. Other than crushing, the single largest cost to produce biomass oils is the production cost of oil seeds. Yeasts, Molds, Fungi and bacteria can be genetically optimized and used to produce oils in closed manufacturing system using inexpensive biomass substrates such as crop residues, wood waste or pyrolysis oil. The oil-oil portion of these organisms can be recycling back into production system, making them truly closed looped.
Performance of biofuels as fuels
Biofuel as fuel has several virtues which include the following (Zhang et., 1998;; NBB, 2002; Shumaker et al., 2003; 2003; Biodoel, EBB, 2005.
a. Biofuels has calorific value of up to 90% that of petroleum diesel and offers comparable engine power;
b. They have higher viscosity, and offers better lubricity than petroleum diesel, hence reduced wear of engine and injector pump;
c. They are non-toxic, has no aromatics and almost no sulphur;
d. They are biodegradable and increases the degradability of biofuel when blended with it, thus any spillage of the fuel does not contaminate the environment;
e. It reduces greenhouse emissions since the CO2 emitted when it is combusted is equal to the amount consumed by the plant during its growth period;
f. They dissipate engine heat better than problem fuel;
g. It does not require engine and fuel system modification before use;
h. Its feedstock is renewable;
i. Bioethanol has high octane rating which prevent engine detonation (knocking) under load;
j. They withstand high compression ratio in an engine which lead to more power per stoke, greater efficiency and better economy.
Research work has generally shown that using biodiesel could dispense with the negative engine conditions encountered by the used of SVOs. Endurance tests performed by Kaufman and Ziejewski (1984) using sunflower methyl ester (ME) in a diesel engine revealed that a 200-hour durability test was successfully completed. Kaufmann et al. (1986) run four tractors on two blends of sunflower ME and petroleum diesel for a period of 7,616 hours (spanning more than three years) and found that bearing wear was normal and there were no power loses or injector and ring coking problems. In another work, two trucks fuelled with biodiesel and operated for a combined distance of 80,467 km showed normal rate of engine wear (Schumacher et al.,). Goodrum et al. (1996) reported better results with peanut ME compared to the qualitative low-sulphur petroleum diesel (D2). Prankl et al. (1999) run 9 vehicles and 1 stationary engine over an interval of 1 – 3 engine oil drains using biodiesel made from rapeseed, sunflower and camelina oils with high iodine numbers and concluded that no unusual deposits were found in the engines.
While the suitability of biodiesel as a fuel is being researched, concurrent studies are also being conducted to determine the quality of the renewable fuel with respect to its emission products.
Biodifuels quality assessment
Biofuels are currently produced in many countries and has been given or conforms to some recognized legislation/standard specifications such as ASTM D6751, EN14214, DIN 51606 and ON C1191 in the US, EU, Germany and Austria respectively. This was done in order to ensure that some minimum standards in terms of regulated emissions are complied with. Several analytical methods are employed in assessing biofuel quality, but the common ones used are chromatographic and spectroscopic methods (Knothe, 2001).
Some studies have been conducted on exhaust emissions of biofuel to determine their health and environmental effects. Schumacher et al. (1992) found that the black smoke produced by diesel engines as they accelerate were reduced by 86% when B100 was used compared to that of petroleum fuel. These researchers as well as Peterson and Reece (1996), Clark and Lyons (1999), Munack et al. (2001) and all reported that exhaust emissions from MEs of rapeseed, soybean and animal fat have lower soot, hydrocarbon, carbon monoxide gum deposit and particulate matter (up to 39% less) concentrations than those of petroleum fuel. Because biofuel does not contain sulphur. It offers lesser chance for acid rain than petroleum fuel due to reduction in sulphur oxide by up to 99%. Even the odour of emissions of biofuel was found to be less offensive than that of nitrogen oxides (NOx), however, there are several reports indicating slightly higher concentrations for biofuel than for petroleum (Clark and Lyons 1999; Munack et al., 2001; Munoz et al., 2004).
Problems associated with biofuels
Apart from the higher NOx in biodiesel emissions, there are a few other reported drawbacks of biodiesel which include the following ( NBB 2002): Fuel ethanol is produced from biologically renewable with the development of sustainable and environmentally sensitive production methods in the agricultural sector, the impact of farming practice is very minimal. The demands for grain to produce fuel ethanol have not resulted in an increased corn or wheat in Canada (Workman, 2007):
a. It degrades natural rubber and plastics
b. Using it entails more frequent changes of fuel filters because it dissolves deposits and sediments in the fuel tank, lines and filters.
c. There is fire hazard if rags soaked with bio-fuel are piled due to oxidation which can cause spontaneous combustion
d. It has a shelf-life of 6 months; hence its production must be tailored to meet demand
e. Because of water in the fuel, it needs a larger tank and jet size in the carburetor
There have been increasing efforts to tackle the challenges posed by and the common problems of bio-fuel. For instance, the challenge perceived by Ortiz-Canavate (1994) regarding the need to standardise bio-fuel before widespread use has been addressed because, as noted earlier, some recognized standards already exist. The first two problems listed above are also being eased out by original equipment manufacturers (OEMs). Similarly, the short shelf-live of bio-fuel is being tackled by attempts of incorporating some additives into the fuel. There are reports showing that some OEMs have even extended the warranties of their products to include bio-fuel usage.
Economics of biofuel production
Biofuel production competes with the food industry because the feedstock used consists of edible oils. Thus, the effect of producing oils for biofuel must be weighed against producing them for food needs or, alternatively, non-edible oils can be produced to serve as more convenient feedstock. Cheaper feedstock such as animal fats and spent or waste frying oils are increasingly being considered as feedstock, since they are claimed to be unhealthy for human and animal consumption due to their trans fatty acids contents. However, these oils must be purified before esterification.
According to Van Gerpen et al. (2004), the dominant factor in bio-fuel production is the cost of feedstock, with capital equipment constituting only 7% of the final product cost. Shlautman (1986) observed that due to low oil yield and production costs, bio-fuel production on commercial basis is possible where government incentives exist. Such incentives are given to feedstock farmers, biofuel producer, markerters and/or users. For instance, the pump price for biodiesel in the US ranges between 2 – 3 times higher than that of petroleum fuel, but in Europe where subsides for biofuel exist, the two fuels have about the same price. For instance, Germany fully exempts biofuels and biofuel blends from motor-fuel tax until 2009 (USDA, 2005)
a. In Brazil where the cost of production is cheaper, the pump price of bioethanol is lower than gasoline (Willian, 2003);
b. A 2002 report by the United State Department of Agriculture concluded that corn ethanol production in the United State a net enegy value of 1.34 mean of 34% more energy was produced than what went in.
In terms of production energy efficiency, however, Richards (2000) reported that cultivation of rapeseed for biodiesel would be more efficient than that of wheat for bioethanol. Kallivroussis et al. (2002) found an energy output/input ratio of 4.5:1 arising from growing sunflower for biodiesel production, while Shumaker et al. (2003) reported that production of biodiesel returns 3.2 units of energy for each unit used in production. Another advantage of biodiesel is the fact that its production by-products (glycerine and fertilizer/feed) have economic value.
Conclusions
Research reports have shown that biodiesel and bioethanol has the same calorific value as fossil based diesel and gasoline and, its combustion products have lesser health hazards and are particulate matter concentrations. The main demerit of the fuel is its higher pump price compared to that of mineral fuel in places where no government incentives for its economical.
There exist some other few surmountable problems of biofuel such as attack on natural rubber and plastics and more frequent changes of fuel filters due to its solvent characteristics. Nevertheless, continued research and favorable policy decisions directed at its production and use make it a potent alternative fuel that might completely replace in the future.
References
[1] Clarke N. N., Lyons D. W., Class 8 truck emissions testing: the effects of test cycles and data on biodiesel operation, Transactions of the ASAE, 42(5), 1999, p. 1211-1219.
[2] Dorado M. P., Arnal J. M., Gomez, Gil A., Lopez F. J., The effect of a waste vegetable oil blend with diesel fuel on engine performance, Transactions of the ASAE, 45(3), 2002, p. 519- 523.
[3] EBB, Biodiesel production statistics for 2004, European Biodiesel Board, Available at: http://www.ebb-eu.org/stats.php., 2005.
[4] Goering C. E., Schwab A. W., Daugherty M. J., Pryde E. H., Heakin A. J., Fuel properties of eleven vegetable oils, Transactions of the SAE, 25(6), 1982, p. 1472 - 1477, 1483.
[5] Goodrum J. W., Patel, McClendon R. W., Diesel injector carboisation by three alternative fuels, Transactions of the ASAE, 39(3), 1996, p. 817 - 821.
[6] Kallivroussis L., Natsis A., Papadakis G., The energy balance of sunflower production for biodiesel in Greece, Biosystems Engineering, 81(3), 2002, p. 347 - 354.
[7] Kaufman K. R., German T. J., Patt, Derry J., Field evaluation of sunflower oil/diesel fuel blends in diesel engines. Transactions of the ASAE, 29(1), 1986, p. 2 - 9.
[8] Knothe, Analytical methods used in the production and fuel quality assessment of biodiesel, Transactions of the ASAE, 44(2), 2001, p. 193 - 200.
[9] Walter J., Bunger M. M., Muller A., Weigel, Gaseous compounds, ozone precursors, particle number and particle size distributions, and mutagenic effects due to biodiesel, Transactions of the ASAE, 44(2), 2001, p. 179 - 191.
[10] Alhasan M., Isah A. G., Garba M. U., Production and Characterization of Biodiesel from Cottonseed Oil, the 6th Annual Engineering Conference S.E.E.T. F.U.T. Minna, 2005.
[11] McDonnel K. P., Ward S. M., Timoney D. J., Hot water degummed rapeseed oil as a fuel for diesel engines, J. Agric. Engng Res., 60, 1995, p. 7 - 14.
[12] Monyem A., Van Gerpen J. H., Canakci M., The effect of timing and oxidation on emissions from biodiesel-fuelled engines, Transactions of the ASAE, 44(1), 2001, p. 35 - 42.
[13] Munack A., Schroeder O., Krahl J., Buenger J., Comrison of relevant exhaust gas emissions from biodiesel and fossil fuel, Agricultural Engineering International, 2001, Available at: http://cigr-ejournal.tamu.edu/Volume3.html.
[14] Munoz M., Moreno F., Morea J., Emissions of an automobile diesel engine fuelled with sunflower methyl ester, Transactions of the ASAE, 47(1), 2004, p. 5 - 11, Available at:
http://www.nbb.org/pdf filesfactsheets/prod quality.pdf.
[15] NBB, Production and testing of ethyl and methyl esters, National Biodiesel Board, 2005, Available at: http://www.nbb.org/resources/reportsdatabase /reports/gen/19941201 gen-005.pdf, accessed 15 June 2005.
[16] Peterson C., Reece D., Emission characteristics of ethyl and methyl ester of rapeseed oil compared to low sulphur diesel control fuel in a chassis dynamometer test of a picking truck, Transactions of the ASAE, 39(3), 1996, p. 805 - 816.
[17] Peterson C. L., Wagner G. L., Auld D. L., Vegetable oil substitutes for diesel fuel, Transactions of the ASAE, 26(2), 1983, p. 322 - 327, p. 332.
[18] Prankl H., Woergetter M., Rathbauer J., Technical performance of vegetable oil methyl esters with a high iodine number, The 4th Biomass Conference of the Americas, 29 August - 2 September, Oakland, California, 1999.
[19] Ricards I. R., Energy bances in the growth of oilseed rape for biodiesel and of wheat for bioethanol, Levington Agriculture Report, 2000, Available at:
http://www.biodiesel.co.uk/levington, accessed 20 April 2005.
[20] Schlautman N. J., Schinstock J. L., Hanna M. A., Unrefined expelled soybean oil performance in a diesel engine, Transactions of the ASAE, 29(1), 1986, 70 - 73, 80.
[21] Shumaker G. A., McKiossick J., Ferland C., Doherty B., A study on the feasibility of biodiesel production in Georgia, 2003, accessed 15 June 2005, Available at:
http://www.agecon.uga.edu/^caed/biodieselrpt.pdf.
[22] USDA, Synthetic diesel may play a significant role as renewable fuel in Germany. Production Estimates and Crop Assessment Division, Foreign Agricultural Service, United States Department of Agriculture, 2005, accessed 2 July 2005, Available at: .
http://www.fas.usda.gov/pecad/highlights/2005/01/bt104/syntheticdiesel.htm.
[23] Van Gerpen J., Shanks B., Pruszko R., Clements D., Knothe G., Biodiesel production technology, 2004, accessed 2 July 2005, Available at: http://www.electricity.com/bio0113003/biodiesel-36244.pdf.
[24] Workman I. T., Cason D. T., Michigan State university ethanol energy balance, United State Department of Agriculture, 2002.
[25] Yong H., Experimental research on cottonseed oil as alternative fuel for single-cylinder diesel engine, AMA, 29(1), 1998, p. 51 - 54.
[26] Zhang X., Peterson C., Reece D., Haws R., Moeller G., Biodegradability of biodiesel in an aquatic environment, Transactions of the ASAE, 41(5), 1998, p. 1423 - 1430.