Determination of Optimal Catalyst Concentration for Maximum Biodiesel Yield from Tigernut (Cyperus Esculentus) Oil
Benjamin Iyenagbe UGHEOKE1, David Onoja PATRICK2, Haruna Mavakumba KEFAS2, Emmanuel Ogo ONCHE1
1Department of Mechanical Engineering, Federal University of Technology, Yola, Nigeria
2Department of Chemical Engineering, Federal University of Technology, Yola, Nigeria
ugheokeb@gmail.com, patodave@yahoo.com, hmkefas@yahoo.com, oncheogo@yahoo.com
Abstract
The profitability of biodiesel production largely depends on the understanding of all the processes involved, especially the optimal values of the transesterification process parameters. In this work, the concentration level of catalyst, potassium hydroxide (KOH) that gives the optimal yield of biodiesel from tigernut (cyperus esculentus) oil was determined. This value was obtained as 0.9 % weight (in gram) of catalyst per volume of transesterified tigernut oil and it gave a biodiesel yield fraction of 0.67.
Keywords
Tigernut oil, Transesterification, Optimal catalyst level, Biodiesel yield
Introduction
There is an increasing campaign for cleaner burning fuel in order to safeguard the environment and protect man from the inhalation of genotoxic substances. The exhaust from petroleum products, especially diesel is known to be toxic and carcinogenous in nature, since they contain polycyclic aromatic hydrocarbons. Apart from these reasons, there has also been a surge in the prices of petroleum products worldwide and it is doubtful if these prices would ever again down-plunge since their rising trend has been consistent since late 2004.
There is therefore a global search for alternative sources of fuel which could be cheaper, safer and more importantly, environmentally friendly. In different parts of the world, depending on availability, diesel has been produced from different biological plants. This was first done as an academic exercise, but today commercialization of this production process and products is on going. Freedman et al (1984) reported the use of fish oil, soy oil, rapeseed oil, cottonseed oil, sunflower, safflower, peanut and linseed oil for the production of methyl esters. According to Barminas et al (2001) categories of suitable agricultural products for biofuel production include seeds, nuts, fruits, leaves, stems and roots, tapped exudates, etc.
Various authors (Batel et al, 1980; Foidl et al, 1996; Darnoko, 1999; Darnoko and Cheryman, 2000 and Mohammed, 2006) have reported the use of transesterification procedures to produce methyl and ethyl esters (biodiesel) from various oils. From their works, one conclusion could be drawn: every oil has its own properties and characteristics and therefore a unique set of transesterification process parameters.
In Nigeria, Barminas et al (2001) have identified a fast growing crop called tigernut (cyperus esculentus) as a source of biofuel. They had used the transesterification process reported by Foidl et al (1996) to produce and thereafter characterize methyl and ethyl esters from tigernut. They however, did not determine the transesterification process parameters for optimal biodiesel production from the nut. Till the time of carrying out this research, the authors had no knowledge that such investigations have been carried out.
This work therefore took as its objective, the optimization of the transesterification process of tigernut oil for biodiesel production. Specifically, the work set out to determine the optimal catalyst concentration level that gives maximum yield of methyl ester (biodiesel) from tigernut (cyperus esculentus) oil.
Materials and Methods
Tigernut Processing
Adequate quantity of mature brown tubers of tigernut (cyperus esculentus) were purchased locally from Jimeta Main Market, Yola. These tubers were washed, sun dried and ground in a manually operated two-disc grinding machine and stored in polyethylene container in a refrigerator.
Oil extraction
The extraction procedure described by Barminas et al (2001) using petroleum ether at between 40-600 C for 12 hours in a sohxlet apparatus was employed to obtain the quantity of tigernut oil used for the transesterification procedure. The transesterification employed in this present work is described below.
Transeterififcation procedure
The bench scale transesterification reactions to produce methyl esters from tigernut oil were carried out in a 1-litre conical flask (hereafter referred to as the reactor), equipped with a thermometer and mounted on a magnetic stirrer hotplate (Stuart Scientific, Britain). The magnetic stirrer was set at a constant speed throughout the experiment, to ensure uniform agitation.
450 ml of the extracted tigernut oil was poured into the reactor and heated to 45º C to improve the oils mix-ability with methanol. The catalyst used is potassium hydroxide (KOH) pellet, analytical grade (Biolab, UK) and was prepared in concentration range of 0.5% w/v to 1.2% w/v, in increments of 0.1% w/v. To achieve the first concentration level, 2.30g of the catalyst (KOH) was dissolved in 100 ml of methanol (GDH Laboratory Supplies, England) and the mixture stirred for 20 minutes, to form potassium methoxide. This potassium methoxide was introduced gently into the heated oil in the reactor and the entire content was brought to a temperature of 55º C at a heating rate of 5º C/min, and then held at his temperature for an hour. The reaction product mixture was allowed to separate into phases by standing for 8 hours in a decantation funnel so as to separate glycerin from the biodiesel. Acetic acid was added to the collected biodiesel, followed by washing with water to remove impurities. The denser soapy mixture was carefully drained from the bottom of the decantation funnel, leaving behind the biodiesel. The volume of the biodiesel obtained was determined in a measuring cylinder.
The procedure described above was repeated for other catalyst concentration levels as shown in table 1.
Results and Discussions
The yield obtained for the various catalyst concentration levels are as shown in the table 1.
Table 1. Yield of biodiesel from tigernut at various catalyst concentration levels
|
Catalyst amount (g) |
Catalyst concentration (% w/v) |
Product volume (ml) |
*Biodiesel yield fraction
|
|
2.30 |
0.5 |
210 |
0.46 |
|
2.75 |
0.6 |
230 |
0.51 |
|
3.20 |
0.7 |
260 |
0.57 |
|
3.65 |
0.8 |
285 |
0.63 |
|
4.10 |
0.9 |
300 |
0.67 |
|
4.55 |
1.0 |
240 |
0.53 |
|
5.00 |
1.1 |
190 |
0.42 |
|
5.45 |
1.2 |
185 |
0.41 |
|
*Each quantity in this column represents average of results obtained from three experiments. |
|||
The catalyst concentration level b was calculated based on the formula:
b = (weight KOH)/(volume oil)*100
Also, biodiesel yield fraction g was obtained from:
g = (Product Volume)/(Oil Volume)
The graphical relationship between the biodiesel yield fraction and catalyst concentration is depicted in figure 1 below.
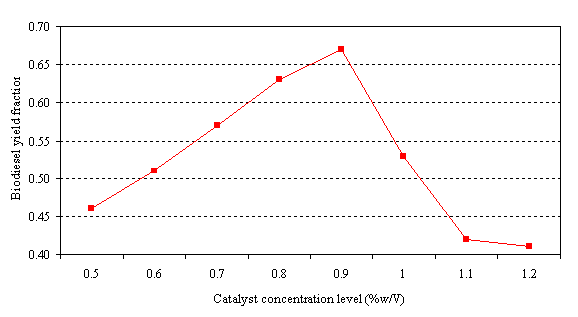
Figure 1. Biodiesel yield versus catalyst concentration
From table 1, it is observed that the product volume increased steadily from 0.5% w/v concentration of the catalyst (KOH) until it peaked at 0.9% w/v of catalyst concentration and thereafter, a decrease was witnessed. It is clear therefore that increment in concentration level of the catalyst would not yield further volume increase in biodiesel obtainable from tigernut. This could be explained from the viewpoint of the reversible nature of the transesterification process as opined by Darnoko (1999). Catalyst concentration levels greater than 1 may have favoured the backward reaction- the formation of glycerine, hence the yield fraction at concentration level of 1.1 is lower than that at concentration level of 0.5 % w/v.
Although, it is possible that variation of other parameters that affect the transesterification process such as (according to Freedman et al, 1984) stirring speed (rate of mixing), molar ratio of methanol to tigernut oil, etc, may affect reaction equilibrium time as well as improve volume yield of biodiesel, it is nonetheless, suspected that variation of these parameters would have a null effect on the optimal catalyst concentration level needed for the transesterification process.
Comparing the results of biodiesel yield obtained from these experiments and the ones obtained elsewhere (Darnoko, 1999) for palm oil as biodiesel resource, it could be inferred that tigernut oil is a viable biodiesel resource and the commercialization of the process of biodiesel production from tigernut oil is not a mirage and may not be far into the future.
Conclusions
The investigation here reported has helped to establish an optimal catalyst concentration level of 0.9% weight in gram (KOH) per volume in milliliter (tigernut oil) in the transesterification process of tigernut (cyperus esculentus) oil. This concentration level gave a biodiesel yield fraction of 0.67.
Further work is required (and the authors are currently working) to fully optimize the overall transesterification process including modelling the reactions and unit operations involved in the production of biodiesel from tigernut oil.
References
[1] Barminas J. T., Maina H. M., Tahir S., Kubmarawa D., Tsware K., (2001), A preliminary investigation into the biofuel characteristics of tigernut (cyperus esculentus), Bioresour. Technol., 79, 2001, p. 87-89.
[2] Batel W., Graef M., Meyer G. J., Moller R., Schroedder F., (1980), Pflonzenole fur die kraftstoff-und energieversorgung, Grundlagen der landtechnik 30(2), 1980, p. 40-51.
[3] Darnoko D., (1999), Continuous production of methlesthers from oil palm and recovery of beta-carotene by membrane technology, PhD thesis, University of Illinois, Urbana, 1999.
[4] Darnoko D., Cheryman M., Kinetics of palm oil transesterification in a batch reactor, JAOCS, 77(12), 2000, p. 1263-1267.
[5] Foidl N., Foidl G., Sanchez M., Mittelbach M., Hackel S., Jatropha Curcas L as a source for the production of biofuel in Nicaragua, Bioresour. Technol., 58, 1996, p.77-82.
[6] Freedman B., Pryde E. H., Mounts T. L., Variables affecting the yield of fatty esters from transesterified vegetable oil, JAOCS, p.1638-1643, 1984.
[7] Mohammed A., Production and characterization of biodiesel from groundnut oil, Proceedings of the 7th Annual Engineering Conference, Federal University of Technology, Minna, Nigeria, 2006.