Spreading of Trisiloxanes EO3 and EO9 Polyethylene Oxide Surfactant over Hydrophobic Substrates
Derrick Ovunda NJOBUENWU1,2
1 Department of Chemical/Petrochemical Engineering, Rivers State University of Science & Technology, PMB 5080 Port Harcourt, Nigeria; 2 Institute of Particle Science & Engineering, School of Process, Environmental & Materials Engineering, University of Leeds, Leeds LS2 9TJ United Kingdom
donadviser@yahoo.co.uk Chedon@leeds.ac.uk
Abstract
The spreading of aqueous dispersions of trisiloxane with various polyethylene oxide chain length surfactant solutions over hydrophobic surfaces is considered from experimental point of view. It is shown that the transfer of surfactant molecules from the droplet onto the hydrophobic surface changes the wetting characteristics in front of the drop making them useful in water-based coatings for plastics and agricultural adjuvant for plant spray. The surfactant molecules increase the solid-vapor interfacial tension and hydrophilize the initially hydrophobic solid substrate just in front of the spreading drop. This process causes water drops to spread over time. The behavior of the drops containing surfactant molecules varied according the surface active molecules that it contains, temperature and the degree of hydrophilicity of the surfactant. The trisiloxane surfactants with polyethylene oxide chain length of nine (EO9) and three EO3 were used on this experiment. Different features of these spreading phenomena were observed. Initial spreading is followed by a period in which the radius of spread increases linearly with time. Water droplets containing the EO9 were more active than the droplets containing the EO3, and they spread more rapidly. On the other hand, the EO3 containing droplets were less active and in most of the experiments they did not spread at all.
Keywords
Spreading, Wetting, Trisiloxane, Surfactant, Hydrophobic solid, Surface tension, Contact angle
Introduction
Surfactant enhanced spreading of aqueous solution on a solid substrate is a basic component in many natural processes and commercial technologies. Some of the applications of the principle include the spreading of liquid droplets such as coatings or inks on substrates ranging from clean metals to plastics [1,2] penetration of inks into porous substrates such as paper [3], spreading of pesticide formulations on waxy weed leaf surfaces [4], and enhanced oil recovery from porous rocks [5]. Most application areas have their own specialized literature and test methods [6].
Surfactants (SURFace ACTive AgeNT) are polar molecules that locate themselves preferentially on material interfaces and lower the surface tension there by their presence. They are active in detergents, emulsifiers, paints, adhesives, inks, alveoli (lungs), wetting agents, foamers, defoamers, herbicides and such likes [7]. The surfactant molecule is an organic compound composed of a polar hydrophilic (water loving) or lipophobic (fat hating) head and a nonpolar hydrophobic (water hating) or lipophilic (fat loving) tail (Figure 1) and is thus semi-soluble in both organic and aqueous solvents [2]. Anticipating our own human ingenuity, nature devised several interesting and vital applications for surface active materials without which our biological systems would not operate, including the very membranes that contain the living cell. Two examples of natural surfactant action closely related to modern applications are the action of bile acids in emulsifying and transporting fatty materials in the digestive process and the action of lung lubricants, which make possible the mechanism of oxygen and carbon dioxide exchange between the lungs and the blood.

Figure 1: The basic chemical nature of surface-active molecules
The physical properties exhibited by surface active agents in aqueous solution, such as solubility, surface tension, interfacial tension, detergency power, foaming capacity, critical micelle concentration, micelle aggregation number, contact angle, spreading coefficient, etc, depend on the composition and structure of the surfactant molecule as a whole and also in part that is, on the composition and structure of the surfactant hydrophobe and hydrophile [8,9]. Hartley [10] considered the possession of hydrophobic and hydrophilic tendencies and their asymmetrical distribution so fundamental a property of surface active agents that he coined the word “amphipathy” for it.
The amphipathic nature of surfactant molecules is responsible for their tendency to concentrate at interfaces (i.e. would prefer to be in neither aqueous nor organic phase) and thereby reduce the free energy of the system in which they interact. The primary mechanism for energy reduction in most cases will be adsorption at various interfaces [11, 12]. However, when all available interfaces are saturated, if there is no more room there, they will congregate together and form micelles. The concentration at which surfactants begin to form micelles is known as the critical micelle concentration, CMC. The overall energy reduction may continue through other mechanisms such as crystallisation, Bilayer and Vesicle Formation.
Organosilicone surfactants are known to increase adhesion (wetting) and spreading of pesticide sprays on foliage due to their high surface activity [4, 13]. They can either improve or reduce the retention of sprays, dependent on their concentration and the target plant/leaf characteristics [14]. The trisiloxane surfactants have the ability to promote spreading, thus, play important role in their use in paints and coatings, personal care products, textiles, the oil industry, and as adjuvant for pesticides [15, 16]. The molecular structure of the trisiloxane surfactants is illustrated in Figure 2. The trisiloxanes are denoted as M(D’EOnR)M, where M represents the trimethylsiloxy group (CH3)3SiO; D’ = -SiCH3R’; R’= (CH2)3; EOn = polyoxyethylene group (OCH2CH2)n; n = average number of the polyoxyethylene group 3, 4, 5 …, R stands for an end-capping group, usually -H, -CH3, or -Ac; Ac = -C(O)CH3.
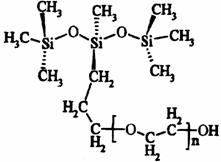
Figure 2. Molecular structure of the trisiloxane polyethylene oxide surfactants M(D’EnOH)M
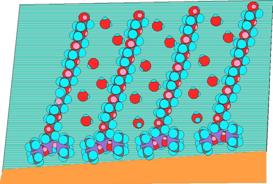
Several works have been done on the spreading of trisiloxanes on solid substrate with their findings reported in literatures with various postulations [3,17-23]. Kumar et al. [24] ATR-FTIR (Attenuated Total Internal Reflection IR Spectroscopy) studies of the adsorption of trisiloxanes and polyoxyethylene surfactants onto model hydrophobic surfaces showed that trisiloxanes superspreading is due to the removal of the high energy water which significantly reduces the large hydrophobic solid/aqueous tension, thus decreasing the contact angle and enhancing spreading. The C12E6 does not remove the high-energy water very effectively, but displaces water further away from the surface. A comparison of these surfactants structure on hydrophobic surfaces is illustrated in Figure 3. Most of the works were concentrated on the EO8 which has being identified as superspreading [24]. In this work, our intention is to provide more understanding on the effect of temperature and trisiloxane surfactant EO9 and EO3 concentration on the modified glass solid substrate.
|
a
|
b
|
|
Figure 3. Structure of Adsorbed Surfactants at the Hydrophobic Solid- Aqueous Interface; (a) EO8, 0.05 wt % in water (~7.5´CAC) (b) C12H6, Conc. of C12E6 = 0.0325 wt% in water (~8´CMC), [24]. For colored print: Red atoms are oxygen; sky blue atoms are oxygen; purple atoms are silicon and gray atoms are carbon; while for black and white print: Dark atoms are oxygen; light atoms are hydrogen; and gray atoms are silicon and carbon |
|
Materials and Methods
Materials
The substrate used in these experiments was formed by a mixture of PMMA (polymethyl methacrylate) and 1,2-Dichloroethane. The compositions were 1% PMMA and 99% 1,2-Dichloroethane. PMMA is a vinyl polymer, made by free radical vinyl polymerisation from the monomer methyl methacrylate. The trisiloxane surfactants were used as obtained from Dow Corning Co., Midland, Michigan, USA without further purification. The polyethylene oxide head of the trisiloxane compounds are monodisperse, and the number given represents only the average tail length. Trisiloxanes of polyethylene oxide chain length 3 and 9 (represented as EO3 and EO9) were used in the experiment. The physical characteristics of trisiloxane surfactants solution are scanty in literature.
Methods
The experimental set up for studying the spreading of surfactant
solutions over hydrophobic substrates shown in Figure 4, consisted of a small
piece made of glass, which was filled with a hydrophobic substrate to the
edges, a chamber where the piece of the glass was placed inside and the
temperature conditions were controlled by either cooling or heating the
chamber, and a video camera connected to a video cassette recorder. Trisiloxane
surfactant mixtures were prepared in glass test tubes using ultra pure water
obtained from a Millipore Ultra Pure water System (Milli-Q plus 185, Millipore
Co., Bedford, WA) with a resistivity of ![]() and a surface tension of 72.2 mN/m at
25°C. Three different trisiloxane surfactant concentrations were studied: 0.01%
wt, 0.1% wt and 0.2% wt at three different temperatures: 6°C, 9.3°C and 25°C.
The small test glass pieces were filled with hydrophobic substrate (mixture of
1% PMMA and 99% 1,2-Dichloroethane) to the edges and put into the oven for
approximately 15-20 minutes until the substrate dried and created a film. The
test glass then was put to the chamber and left for approximately 15 minutes to
reach the same temperature as the chamber. A water reservoir, with two pipes
was connected to the chamber and circulated around it for temperature control.
The syringe was filled with the surfactant solution and the needle was passing
through a small hole of the chamber, so only the needle was in the inlet of the
chamber.
and a surface tension of 72.2 mN/m at
25°C. Three different trisiloxane surfactant concentrations were studied: 0.01%
wt, 0.1% wt and 0.2% wt at three different temperatures: 6°C, 9.3°C and 25°C.
The small test glass pieces were filled with hydrophobic substrate (mixture of
1% PMMA and 99% 1,2-Dichloroethane) to the edges and put into the oven for
approximately 15-20 minutes until the substrate dried and created a film. The
test glass then was put to the chamber and left for approximately 15 minutes to
reach the same temperature as the chamber. A water reservoir, with two pipes
was connected to the chamber and circulated around it for temperature control.
The syringe was filled with the surfactant solution and the needle was passing
through a small hole of the chamber, so only the needle was in the inlet of the
chamber.
The spreading experiments were conducted by producing a drop of surfactants solution from the syringe such that it hung from the tip of the needle. The drop was then gently allowed to drop on the surface of the glass coated with the substrate film. On touching the substrate film surface, the surfactant solution spreads on it for about two minutes. The expanding drop was viewed in transmitted light and captured using a CCD – videotape system. The spreading event was recorded onto video tape in VHS format. The video tape record was digitized into avi files using personal-computer with Pinnacle Studio 9 software at 25 frames/sec and at 640 x 480 pixel resolution. The avi files were converted to frames using frame grabber irfan View software. The digitized movie was analyzed frame-by-frame using image analysis software, Scion Image to determine the area covered by the spreading droplet as a function of time.
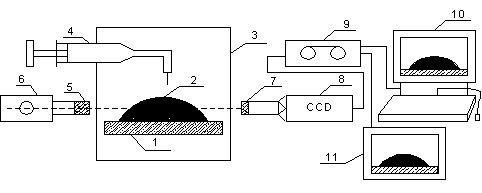
Figure 4. Experimental set-up: 1-hydrophobic substrate, 2-liquid drop, 3-hermetically closed, thermo stated chamber, 4-syringe, 5-light source, 6-collimating lens, 7-tele-photo objective, 8-CCD camera, 9-Tape Recorder, 10-Personal Computer and 11-Television.
Results and Discussion
The spreading of trisiloxanes over hydrophobic solid substrates was investigated. Typical image frames acquired by the image analysis system described in the experimental procedure (see Figure 4) are displayed in Figure 5. The figure shows a printout of samples of experimental measurement for trisiloxanes EO3 at concentration 0.2% wt at 25°C spreading on hydrophobic surface after 0.04 sec and 90 sec, and EO9 at concentration 0.1% wt at 25°C spreading on hydrophobic surface after 0.04 sec and 90 sec respectively. Similar images (framed) were grabbed by the image analyser for other concentration and temperatures from which the radius of spreading for spreading and contact angle for trisiloxanes spreading on solid substrate were digitalised and measured using software as a function of time. Those images have a similar trend to the images in Figure 5 and thus, are not shown. The results from the analysis were shown in Table 1.
|
a |
b |
|
c |
d |
|
Figure 5. Trisiloxanes Solution spreading on hydrophobic solid substrate various concentrations and temperatures. (a) EO3 0.2% wt at 25°C after 0.04 sec; (b) EO3 0.2% wt at 25°C after 90 sec;. (c) 0.1% wt at 25°C in 0.04 sec. (d) EO9 0.1% wt at 25°C in 90 sec |
|
Table 1. Experimental data
|
Surfactant |
EO3 |
EO9 |
EO9 |
EO3 |
EO3 |
EO9 |
EO3 |
EO3 |
EO9 |
EO9 |
|
Conc. (%wt) |
0.1 |
0.1 |
0.1 |
0.01 |
0.01 |
0.01 |
0.2 |
0.2 |
0.2 |
0.2 |
|
Temp. °C |
9.3 |
9.3 |
25 |
9.3 |
25 |
25 |
6 |
25 |
6 |
25 |
|
Ri, (cm) |
0.48 |
0.745 |
0.485 |
0.18 |
0.38 |
0.42 |
0.49 |
0.425 |
0.63 |
0.595 |
|
Rf, (cm) |
0.535 |
1.045 |
0.925 |
0.195 |
0.415 |
0.625 |
0.525 |
0.435 |
1.11 |
0.965 |
|
θi°, (degree) |
63.14 |
35.015 |
52.657 |
88.388 |
75.589 |
77.983 |
53.131 |
57.881 |
40.913 |
28.075 |
|
θf°, (degree) |
54.409 |
13.642 |
6.806 |
80.586 |
89.315 |
25.2521 |
44.541 |
50.467 |
6.703 |
30.081 |
According to the observations, in most of the runs, the behavior of the droplets was either exhibiting spreading or not spreading. Different kinetics of spreading was also observed for the EO3 and EO9 and at various temperatures. It is interesting to note that the droplet containing the EO9 and EO3 surfactant and at high temperatures spread relatively faster than the same droplets at low temperatures. There was initial fast spread followed by the second stage which is slower. Figure 6 shows the observed spreading radius of trisiloxane solution droplets over PMMA film at 0.2%wt concentration of EO3 and EO9 and at temperatures of 6 and 25oC.
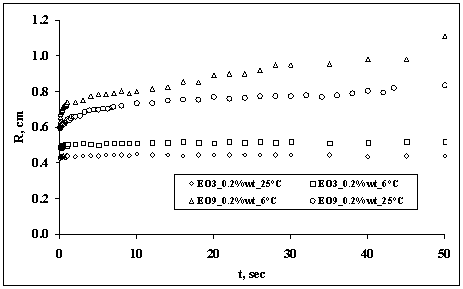
Figure 6. Spreading of a water droplet containing 0.2%wt concentration of E03 and E09 at different temperature
In Figure 7 it can be seen a similar plot for droplets containing EO3 and EO9 of 0.1%wt concentration at 9.3 and 25oC.
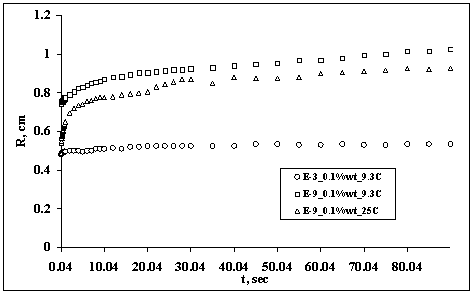
Figure 7. Spreading of a water droplet containing 0.1%wt concentration of E03 and E09 at different temperature
In both figures it was observed that the droplets containing the EO9 spread faster than the droplets containing EO3. By comparing Figures 6 and 7, the effect of concentration of surfactant and temperature on the droplet spreading is evident. In the case of the 0.1%wt concentration of the surfactant, it can be seen that at 25°C the droplet is spreading very fast in addition to that at 9.3°C. The behavior of the EO3 containing-droplet was observed not to spread at all.
Conclusion
The behavior of drops containing surfactant molecules is varying according the surface active molecules that it contains. The surfactants EO9 and EO3 were used on this experiment. Most of the results of the experiment were satisfactorily. Water droplets containing the EO9 were more active than the droplets containing the EO3, and they spread more rapidly. On the other hand, the EO3 containing droplets were less active and in most of the experiments they did not spread at all. In comparison to other non-ionic hydrocarbon surfactant, aqueous dispersions of trisiloxane polyethylene oxide surfactants spread rapidly over hydrophobic surfaces making them useful in water based coatings for plastics and in agricultural adjuvant.
References
[1] Asthana R., Sobczak N., Wettability, Spreading, and Interfacial Phenomena in High-Temperature Coatings, JOM-e, 52(1), 2000.
[2] Njobuenwu D. O., The Role of Surfactants in Coatings: Application of Colloid Engineering, Global J. Mech. Eng., 2005, 6(1), p. 22-27.
[3] von Bahr M., Wetting and Capillary Flow of Surfactant Solutions and Inks, PhD Thesis, Lund University, Sweden, 2003.
[4] Stevens P. J. G., Organosilicone Surfactants as Adjuvants for Agrochemicals, Pesticide Sci., 1993, 38, p. 103-22.
[5] De Coninck J.; de Ruijter M., Voue M., Dynamics of Wetting, Current Opinion in Colloid and Interface Sci., 2001, 6, p. 49-53.
[6] Hill R. M., Dynamics of Surfactant Enhanced Spreading, ECJ European Coatings Journal, 1998, 7-8, p. 550-553.
[7] Moroi Y., Micelles: Theoretical and Applied Aspects, Plenum Press, New York, 1992.
[8] Schwarz E. G., Reid W. G., Surface Active Agents - Their Behavior, Ind. Eng. Chem., 1964, 56, p. 26-31.
[9] Myers D., Surfactant Science and Technology, VCH Publishers, Inc, USA, 1998.
[10] Hartley G. S., Solutions of Soap-Like Substances, In: Progress in the Chem. of Fat and other Lipids, Pergamon Press London, 1955, 3, p.20-55.
[11] Jaycock M. J., Parfitt G. D., Chemistry of Interfaces, Ellis Horwood, Chichester, 1981.
[12] Starov V. M., Kosvintsev S. R., Velarde M. G., Spreading of Surfactant Solutions over Hydrophobic Substrates, J. Colloid Interface Sci., 2000, 227, 185-190.
[13] Zabkiewicz J. A., Coupland D., Ede F., Effects of surfactants on droplet spreading and drying rates in relation to foliar uptake, In: Pesticide Formulations; Innovations and Developments, B. Cross and H.B. Scher (Eds); ACS Symposium Series 371, 1988, p. 77-89.
[14] Holloway P. J., Physicochemical Factors Influencing the Adjuvant-Enhanced Spray Deposition and Coverage of Foliage-Applied Agrochemicals, In: Interactions between Adjuvants, Agrochemicals and Target Organisms by Holloway P. J. G., Rees R. T., Stock D. (Eds); Ernst Schering Research Foundation Workshop 12, Springer-Verlag, Berlin, 1994, p. 83-106.
[15] Hill R. M., Superspreading, Current Opinion in Colloid and Interface Science, 1998, 3(3), p. 247-254.
[16] Ananthapadmanabhan K., Goddard E., Chandar P. A., Study of the Solution, Interfacial and Wetting Properties of Silicone Surfactants, Colloids Surf., 1990, 44, p. 281-297.
[17] Zhu X., Miller W., Scriven L., Davis H., Superspreading of Water-Silicone Surfactant on Hydrophobic Surfaces,” Colloids Surf. A, 1994, 90, p.63-78.
[18] Rosen M. J., Song L. D., Superspreading, Skein Wetting, and Dynamic Surface Tension, Langmuir, 1996, 12, p. 4945-4949.
[19] Svitova T. F., Hoffmann H., Hill R. M., Trisiloxane Surfactants: Surface/Interfacial Tension Dynamics and Spreading on Hydrophobic Surfaces, Langmuir, 1996, 12, p. 1712-1721.
[20] Stoebe T., Hill R. M, Ward M. D., Davis H. T., Enhanced Spreading of Aqueous Films Containing Ionic Surfactants on Solid Substrates, Langmuir, 1997, 13, p. 7276-7281.
[21] Stoebe T., Lin Z., Hill R. M., Ward M. D., Davis H. T., Enhanced Spreading of Aqueous Films Containing Ethoxylated Alcohol Surfactants on Solid Substrates, Langmuir, 1997, 13, p. 7270-7275.
[22] von Bahr M., Tiberg F., Yaminsky V., Spreading Dynamics of Liquids and surfactant Solutions on Partially Wettable Hydrophobic Substrates, Colloids Surf. A, 2001, 193, p.85-96.
[23] Churaev N. V., Esipova N. E., Hill R. M., Sobolev V. D., Starov V. M., Zorin Z. M., The Superspreading Effect of Trisiloxane Surfactant Solutions, Langmuir, 2001, 17(5), p. 1338-1348.
[24] Kumar N., Steiner C., Maldarelli C., Couzis A., A Comparative Study of the Adsorption of Trisiloxane and Polyethylene Glycol Ethers at the Hydrophobic Solid-Aqueous Interfaces, AIChE National Meeting, Dallas, Texas, 1999.
Appendix
Sample Calculations
Example given for surfactant EO3 with concentration 0.2% wt at 25°C For the given data of coordinates obtained using the scion image software on the frame grabbed:
|
Points |
X |
Y |
|
1 |
0.66 |
1.19 |
|
2 |
1.07 |
0.95 |
|
3 |
1.51 |
1.18 |
For Radius of spread R(t) in cm:
R(t) = (X3 – X2)/2 = (1.51 – 0.66)/2 = 0.425
For Height h in cm:
H = [(Y3 – Y1)/2] – Y2 = [(1.18 + 1.19)/2] – 0.95 = 0.235
For Sinθ in rad:
Sinθ = (2 ´ R ´ h)/(R2 + h2) = (2 ´ 0.425 ´ 0.235)/(0.4252 + 0.2352) = 0.8469
For θ in rad:
θ = sin-1 θ = sin-1(0.8469) = 1.0101
For θ in degree:
θ = 57.881