Corrosion Inhibition of Titanium in Artificial Saliva Containing Fluoride
Latifa KINANI and Abdelilah CHTAINI*
Equipe d’Electrochimie Moléculaire et Matériaux Inorganiques, Maroc
chtainia@yahoo.fr (*Corresponding authors)
Abstract
The objective of this study was to demonstrate the effect of eugenol on the titanium corrosion in artificial saliva enriched with eugenol at different concentration. The corrosion behaviour and titanium surface characterization were investigated by electrochemical measurements and SEM.
Keywords
Corrosion; Titanium; Fluoride; pH; Eugenol; SEM.
Introduction
Titanium and its alloys are widely used in dentistry as prosthetic appliances because of a high corrosion resistance and good biocompatibility. These valuable properties are caused by passive films that are rapidly formed in the body fluid environment [1-3]. Recently, mouth-rinses, toothpastes, and prophylactic agents containing fluoride are utilizing to prevent the dental caries. However, decreasing the corrosion resistance of titanium in solutions containing fluoride has been reported [4-12]. The corrosion behaviours are related to the concentration of fluoride and the surrounding environment Therefore, the surface reaction on titanium under the existence of fluoride is important to understand the corrosion and tarnish of titanium in oral environment. Recently the corrosion of titanium was suppressed in solutions containing fluoride and eugenol (Figure1).
Eugenol occurs widely as a component of essential oils and is a major constituent of clove oil. It has been used since at least the nineteenth century, primarily as a flavouring agent, in a variety of foods and pharmaceutical products, and as an analgesic in dental materials. Recently it has been used as inhibitor on the corrosion of stainless steel in phosphoric acid solution [13].
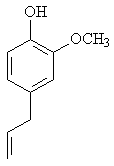
Figure 1. Chemical formula of eugenol
Materials and Methods
The specimens used were plats (1cm × 1cm × 1mm) placed in different medium, adaptable to the working electrode. The samples were mechanically cleaned with abrasive strips. The electrolyte reference used was Fusayama Meyer artificial saliva. The composition of this solution, which closely resembles natural saliva, is KCl (0.4 g/l), NaCl (0.4 g/l), CaCl2. 2H2O (0.906 g/l), NaH2PO4, 2H2O (0.690 g/l), Na2S, 9H2O (0.005 g/l), Urea (1 g/l). The pH was measured with an XC type glass electrode. The pH (7.03) of this reference saliva corresponding to our first test medium. The second medium used had the same contents as the first, but the pH was lowered by adding lactic acid. This acid was chosen in order to obtain conditions that were as close as possible to the clinical reality. The third medium was identical to the reference medium but was enriched with fluoride ions with a concentration of 0.1% and the last medium is containing fluoride at pH = 2 at different concentration of eugenol.
For electrochemical step, we used a glass electrochemical cell, with the thermostat set at 37 ± 0.1°C. The three electrodes system was used, with a saturated calomel electrode, platinum plat (1cm × 1cm) counter electrode and working electrode (specimens test) connected to a Voltalab 10 type computer controlled potentiostat. The electrochemical analysis involved, measuring of linear polarization resistance (Rp), potentiodynamic polarization and AC impedance in different media. The surfaces of the specimens were observed on scanning electron microscopy.
Statistical analyses
The corrosion parameters, including Icorr and Rp, were statistically analyzed using one - way ANOVA for analyzing the factor of NaF or eugenol concentration in 0.1% NaF containing medium. The test of Tukey’s test (α = 0.05) was chosen as the following multiple comparison technique when necessary.
The results of two-way ANOVA for Rp values and Icorr proved that fluoride, and the concentration of the eugenol in presence of fluoride had a statistically significant influence on the value of Rp(p < 0.00002) and I corr (p < 2.109010-8).
Results and Discussion
The electrochemical parameters determined from the polarization curves (Figure 2) are given in Table.1. The corrosion current density of titanium grade2 increase with increasing of pH, from 0.013 mA/cm2 in reference medium to 0,445 mA/cm2 in pH 2 medium and 2.013 mA/cm2in medium saliva pH 2 containing fluoride.
Our results have shown that fluoride ions could cause the breakdown of the protective passivation layer, so it’s necessary to church inhibitory that suppressed this corrosion.
Table1. Summary of electrochemical parameters for titanium grade 2 in different media
|
Electrolyte |
Icorr(mA/cm2) |
Rp(Ω∙cm2) |
E(I=0)mV |
|
Medium saliva pH=7.03 |
0.013 |
5220 |
-795.3 |
|
Medium saliva pH=2 |
0.445 |
207.26 |
-1488 |
|
Medium saliva pH=2+fluoride |
2.013 |
66..5 |
-1520 |
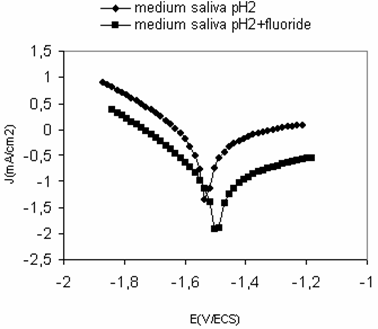
Figure 2. Polarization curves after 2h of immersion in medium saliva pH 2 with and without fluoride
The electrochemical parameters determined from the polarization curves (Figure3) are given in Table 2.
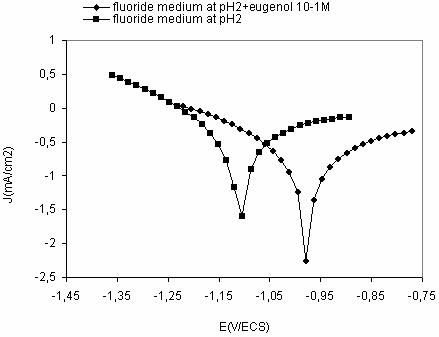
Figure3. Polarization curves after 2h of immersion in fluoride medium saliva pH2 with and without eugenol
Table 2. Summary of electrochemical parameters for titanium grade 2 in fluoride medium at different Concentration of eugenol
|
Concentration of eugenol |
I corr (mA/cm2) |
Rp(Ω∙cm2) |
E(I=0) |
E% |
|
0 |
2.013 |
66.5 |
|
|
|
10-5 M |
0.538 |
164.03 |
-1422.5 |
73.273 |
|
10-3 M |
0.167 |
434.16 |
-1501.9 |
91.703 |
|
10-1 M |
0.059 |
1610 |
-1062.6 |
97.069 |
The corrosion current density of titanium grade 2 decrease with increasing concentration of eugenol, from 2,013 mA/cm2 in reference medium at pH2 containing fluoride (0,1%) to 0,059 mA/cm2 in 10-1M concentration of eugenol medium. The results showed that eugenol revealed a good corrosion inhibitor. The inhibition efficiency depends the concentrations of eugenol.
An impedance spectroscopy study was performed in order to confirm the results obtained with polarization tests. Figure 4 shown the impedance diagrams recorded for titanium grade 2 in different medium to examine the concentration of eugenol effect. In case fluoride medium at pH2 the impedance curve is in the form of a half-circle which can be attributed to electron transfer step. The diameter of circle increased with increasing the concentration of eugenol. However, at concentration10-1M of eugenol (Figure 5) the diagrams curve is in the form of three-circle corresponding the formation the tridimentionel film on the surface that suppressed corrosion.
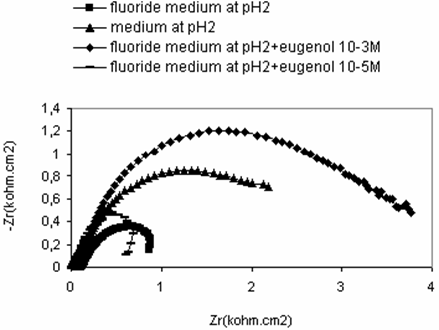
Figure 4. Electrochemical impedance spectroscopy for titanium grade 2 in different saliva media
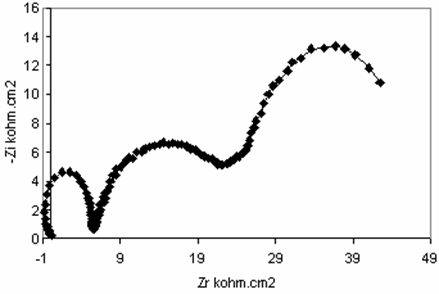
Figure 5. Electrochemical impedance spectroscopy for titanium grade 2 fluoride media at pH2 + (10-1M) eugenol
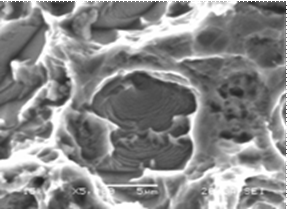
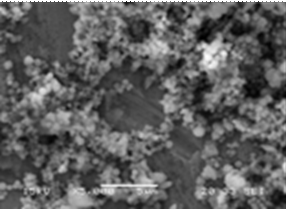
The SEM micrographs (Figure 6) of titanium grade 2 after electrochemical analyses confirmed the electrochemical results They show a general change in the surface of pure titanium in Fusayama Meyer saliva medium, in the much more aggressive fluoridated-acidified saliva medium the metal is characterized by localized pitting. However, the addition of eugenol in fluoride medium at pH2 led the formation of film on the surface which increases the metal resistance.
|
|
|
|
(a) |
(b) |
Figure 6. SEM of titanium after test electrochemical a) fluoride media at pH2, b) fluoride media at pH = 2+eugenol
Conclusion
Behaviour of titanium in a solution containing fluoride and eugenol were studied electrochemical properties, surface analyses by SEM and pH. These results are summarized as following:
1. The corrosion resistance of titanium specimens solution containing. Fluoride decreased in comparison with that in a solution not containing fluoride. Adding eugenol to the solution resulted in preventing the corrosion of the titanium from the fluoride.
2. The rate corrosion of titanium in acidic medium saliva (pH =2) was clearly lower than that in the solution containing fluoride at pH = 2.
From these results, the effects of eugenol are not only the protection of titanium from fluoride attack but also the suppression of dissolution of titanium ions via formation of the eugenol films. These effects evidently suppress the corrosion of titanium by fluoride.
References
1. Hanawa T., Ota M., Characterization of surface .lm formed on titanium in electrolyte usingXPS, Appl Surf Sci, 1992, 55, p. 269-276.
2. Ong J. L., Lucas L. C., Raikar G.N., Connatser R., Gregory J. C., Spectroscopic characterization of passivated titanium in a physiologic solution, J Mater Sci Mater Med, 1995, 6, p. 1131-119.
3. Hanawa T., Asami K., Asaoka K., Repassivation of titanium and surface oxide .lm regenerated in simulated bioliquid, J Biomed Mater Res, 1998, 40, p. 530-538.
4. Lausmaa J., Kasemo B., Hansson S., Accelerated oxide grown on titanium implants duringautoclavin g caused by uorine contamination, Biomaterials, 1985, 6, p. 23-27.
5. Wilhelmsen W., Grande A. P., The in.uence of hydro.uoric acid and .uoride ion on the corrosion and passive behavior of titanium, Electrochim Acta, 1987, 32(10), p. 1469-1472.
6. Boere G., Influence of .uoride on titanium in an acidic environment measured by polarization resistance technique, J Appl Biomater, 1995, 6, p. 283-288.
7. Oda Y., Kawada E., Yoshinari M., Hasegawa K., Okabe T., The in.uence of .uoride concentration on the corrosion of titanium and titanium alloys, Jpn J Dent Mater, 1996, 15, p. 317-322.
8. Reclaru L., Meyer J.-M., Effects of fluorides on titanium and other dental alloys in dentistry, Biomaterials 1998, 19, p. 85-92.
9. Nakagawa M., Matsuya S., Shiraishi T., Ohta M., Effect of .fluoride concentration and pH on corrosion behavior of titanium for dental use, J Dent Res, 1999, 78(9), p. 1568-1572.
10. Nakagawa M., Matsuya S., Udoh K., Corrosion behavior of pure titanium and titanium alloys in .uoride-containingsolutions, Dent Mater J, 2001, 20(4), p. 305-314.
11. Nakagawa M., Matsuya S., Udoh K., Effect of fluoride and dissolved oxygen concentrations on the corrosion behaviour of pure titanium and titanium alloys, Dent Mater J, 2002, 21(2), p. 83-92.
12. Schiff N., Grosgogeat B., Lissac M., Dalard F., Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys, Biomaterials, 2002, 23, p. 1995-2002.