Spreading behaviour of silicone oil and glycerol drops on coated papers
Mohammad Ilyas KHAN1, Mohamed Mahmoud NASEF2
Faculty of Chemical and Natural Resources Engineering, Universiti Teknologi Malaysia, 81310 UTM, Skudai, Johor, Malaysia
1 Department of Polymer Engineering, m.i.khan@fkkksa.utm.my, corresponding author
2 Department of Chemical Engineering, mahmoudeithar@fkkksa.utm.my
Abstract
The effect of physical properties represented by viscosity and surface tension of liquid spreading on coated papers was investigated. Two substrates of different surface roughness were used to study the spreading behaviour of silicon oil and glycerine/water solutions in terms of contact angle, wetted drop base area and drop height as a function of time. The liquid spreading on coated papers was found to vary depending on the liquid physical properties. Liquids with lower surface tension (silicon oil) and viscosity prevailed better wettability and vice versa. High surface roughness reduced the liquid spreading. Despite being traditionally used as a wetting indicator, contact angles were found to be insufficient to evaluate the spreading of liquids on these substrates. Hence, other parameters such as drop base area and drop height should also be considered.
Keywords
Liquid spreading, Viscosity, Surface tension, Contact angle, Surface roughness, Drop base area, Drop height
Introduction
Liquid spreading behaviour on solid surfaces is increasingly becoming popular and the subject of a large number of investigations in the recent years. Liquid spreading is a process that is being utilized in a large number of industrial applications despite being a complex phenomena [1]. There are two important parameters characterising the spreading of liquid drops: the degree of wetting and the rate of spreading. Contact angle measurements are commonly used to evaluate the degree of spreading/wettability of a surface by a liquid. Various examples are available in which complete wetting of surfaces may be required e.g. membrane separation, photographic and speciality paper coating, adhesives, printing, spraying of pesticides and herbicides (agriculture applications). On the other hand, some applications may require a high degree of non-wetting (high liquid repellence) e.g. water proof coating materials and in micro-fluidic devices [2, 3].
Liquid spreading is affected by various factors. The important factors are substrate properties (surface roughness and its geometry i.e. pattern and chemical make-up composition) and system conditions such as temperature and humidity and liquid properties namely viscosity, surface tension and density [1]. Among liquid properties, viscosity was found to have a pronounced effect in a form of resistance to spreading of liquid on solid surfaces and therefore, spreading of highly viscous liquids tend be lower than that of liquids with low viscosity [4, 5].
The effect of roughness on spreading of non-volatile liquids on rough surfaces was studied by Cazabat and Stuart [6]. It was found that wetting of a smooth substrate is governed by two regimes: capillary and gravity, unlike rough surfaces which have an additional regime caused by liquid quick spreading in the roughness of the surface till it is consumed by troughs and valleys on the surface. Similar study was conducted by Kandlikar and Steinke [7] who concluded that the contact angle decreased with increasing roughness up to a critical value beyond which it increases with further increase in roughness.
Materials of high surface energy such as glass show an increase in the contact angles with the decrease in the surface tension of the spreading solutions. Unlikely materials of low surface energy like PTFE produces lower contact angles i.e. better wetting by reducing the liquid surface tension. Therefore, most of previous studies introduced surfactants to the wetting liquids to reduce the surface tension and as a result achieve good wettability [8-10]. Other studies such as Sikalo et al [4] reported that a small drop of liquid of small surface tension splashes more while impacting on a smooth plate than a drop of high surface tension does. Xiao-dong et al. [11] measured the static contact angles of silicone oil on glass, aluminium and stainless steel plates using the sessile drop method and reported that silicone oil is completely wetting all three surfaces. Also complete wetting of acrylic solid substrate with silicone oil was recently studied by Njobuenwu [12]. However, in these studies only contact angle measurements were used, which is presumably not sufficient to evaluate the degree of wettability. In the present study, the wetting behaviour is investigated in terms of contact angle, drop base area and drop height evolution with time towards better evaluation of wetting characteristics. The selection of such parameters would certainly give an insight on the solid/liquid interactions towards better understanding of the spreading behaviour of liquids. To achieve this objective, two paper substrates were wetted with glycerine/water solutions (glycerol) and with silicone oils while a drop shape analysis technique was applied using the FTA 188 videotensiometer.
Material and Method
The Apparatus
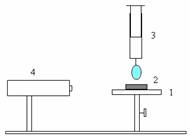
A simplified system (schematised in Fig.1) consists of an adjustable horizontal plate (marked as 1) to host the test substrate (marked as 2) and a syringe (marked as 3) to deliver liquid drops on the solid surface.

(a) (b)
Figure 1. (a) Schematic representation of experimental rig; (b) Contact angle on the solid surface
The process of spreading of the liquid drop is captured using a high-speed video camera (marked as 4) mounted parallel to the drop. All measurements were carried out at room temperature. Images of spreading drops were analysed for contact angle, drop base area, and drop height, volume and maximum diameter in correlation with time by the built-in software in the computer system. Each experiment was repeated three times and the results presented here are the averaged data for each experiment. Figure 1b also shows contact angle image taken for glycerine/water drop resting on one of the solid substrate.
The Coating Fluids
Four sets of liquids including 2 sets of silicone oils (Basildon Chemicals, UK) and 2 sets of glycerine/water solutions were used. The viscosity of all fluids were measured to an accuracy of ± 5% in a Bohlin CVO viscometer equipped with a Peltier system allowing an accurate control of the sample temperature (± 0.1°C). The surface tensions of all fluids were measured to an accuracy of ± 2 % with a FTA 188 video tensiometer using the pendent drop method. All measurements were conducted at room temperature according to the procedure described elsewhere [13]. The surface tension of 2 sets of silicone oil was found to be 20.0 mN/m and the viscosities were 49 and 519 mPa, respectively. For the two sets of glycerine-water solutions, the surface tension was found to be 65 mN/m and the viscosities were 54 and 643 mPa·s, respectively. The physical properties of all fluids used in this work are summarized in Table 1.
Table 1: Measured physical properties of the test solutions used
|
Sample |
Glycerine/water set 1 |
Glycerine/water set 2 |
Silicone oil set 1 |
Silicone oil set 2 |
|
r (kg·m3) |
1203 |
1254 |
951 |
963 |
|
s (mN/m) |
65 |
65 |
20 |
20 |
|
m (mPa·s) |
49 |
643 |
54 |
519 |
The Substrates
Substrates were solid photographic base materials supplied by Ilford Imaging (Switzerland). Surface roughness measurements were carried out using the Veeco-WYKO optical profiling system. A small sample (» 3 x 5 cm) of the solid paper was sandwiched between two glass slides. The sample was then mounted onto the adjustable rack and the scanning was carried out. Figure 2 shows surface profiles of the substrates measured with the WYKO surface profiling system. The average roughness (Ra) is found to be 199.51 and 539.56 nm for substrate 1 and 2, respectively.
|
Substrate 1, Ra = 199.51 nm; Substrate 2, Ra = 539.56 nm |
Figure 2. Surface profiles of the substrates measured with the Veeco, WYKO surface profiling system
Results and Discussions
Effect of Contact Angle
Figures 3 (a and b) show the variation in the contact angle with time for both substrates (1 and 2) with 4 sets of liquids: 2 sets of glycerine/water solutions, respectively denoted as GWS1 and GWS2 and 2 sets of silicone oils numerated as SOS1 and SOS2, respectively. In both cases, the initial contact angle recorded high values, which decreases rapidly after making the initial contact on the substrate, reaching equilibrium after a short period of time. For instance, the drop spreading with GWS1 and GWS2 reached an equilibrium contact angle of 70° and 60° after approximately 15 seconds beyond which no further spreading could be observed. This indicates that although the contact angle values are different, the wetting behaviour of the two substrates is otherwise similar. On the other hand, the contact angles with SOS1 reached almost zero within 5 and 9 seconds with substrate 1 and 2, respectively. Again, the spreading curves seem to be very similar for both SOS1 and SOS2.
However, the contact angles were found to be larger with SOS2 and approaches an almost equilibrium state in a longer time (20 seconds) compared to SOS1. This behaviour is due to the viscosity effect i.e. liquids with higher viscosity tend to have higher resistance to spreading than those of lower viscosity because higher viscous dissipation reduces the rate of spreading [1]. This observation is in a good agreement with previous studies conducted by [4, 5]. Somehow, the effect of viscosity was not profound with GWS1 and GWS2 as indicated the close values of the equilibrium contact angles of both liquids with the two substrates.
The effect of surface tension of liquids on spreading on the substrates 1 and 2 can be also observed clearly in Figure 3 (a and b). The decrease in the initial contact angle for liquids of lower surface tension (SOS1 and SOS1) is sharper than that of liquid of high surface tension (GWS1 and GWS2) reflecting that wetting with glycerine/water mixtures is not spontaneous as compared to wetting with silicone oils. This observation emphasize on the role of interfacial tension in affecting wettability. Therefore, the use of surfactants becomes a common practice to enhance spreading and wettability by reducing surface tension of the liquids. However, the surfactant concentration must be kept below the critical micelle concentration beyond which the spreading is hindered by the formation micelle structure. For examples Ghannam used Triton X-100 to enhance the spreading of crude oil droplets over limestone [8]. Other surfactants such as glucoseamide-based trisiloxane [9] and pentaethylene glycol monododecyl ether [10] and the spreading of trisiloxane surfactants over hydrophobic substrates [14] are also used to enhance the spreading of liquids.
The surface roughness of the substrate is also found to play a significant role in affecting the liquids spreading as shown the decreasing trends of 4 sets liquids on substrate 1 (Figure 3a) which is smoother than substrate 2 (Figure 3b). For instance, the spreading of all tested liquid is delayed with the increase of roughness. The spreading of SOS1 is delayed by 4 seconds with the increase of roughness from 199.51 to 539.56nm indicating that achieving an equilibrium contact angle with rough surfaces requires longer time i.e. the velocity of the wetting line seems to be strongly dependent on the surface roughness.
Surface topography is known to affect wetting behaviour. The measured macroscopic equilibrium contact angles depend on both the chemical nature of the surface and the local surface geometry. Unlike wetting of a smooth surface, the effect of surface roughness on dynamic wetting is not well established and is still a subject of debate. Nonetheless, [15] presented a theory to describe the wetting phenomena and the contact line de-pinning as a function of microstructure of rough surfaces using noise correlation functions. The relationship between heterogeneous surfaces and the dynamic contact angle was theoretically addressed by [16] based on Frenkel’s approach of free energy changes and viscous dissipation.
The contact angle of wetting of smooth and rough heterogeneous surfaces prepared on glass slides by organic liquid was experimentally determined by [17]. The advancing and receding contact angles were measured and plotted against contact angle hysteresis. By extrapolating the contact angle variation curve to zero hysteresis, equilibrium contact angle was obtained. However, their observation contradicts the Wenzel's result. The effect of surface roughness and surface temperature on dynamic contact angle of de-ionised water on copper and stainless steel surfaces was investigated by [7] using a photographic technique. The authors reported that the contact angle decreased with increasing surface roughness up to a critical value of roughness. Further increase in roughness resulted in the increase in contact angle.
The effect of surface roughness on wetting of smooth and rough glass surfaces with liquids (water, isopropanol and glycerine) was also reported by [4]. Their investigations were conducted on the impact of droplets on horizontal surface at various heights. It was found that the wettability of glass decreased significantly on a rough topography when water impacted on the surface unlike isopropanol, which showed zero static contact angles regardless of surface roughness. The maximum spread and spreading rate for the rough glass were lower than those for the smooth surface. Cazabat and Stuart [6] studied the effect of surface roughness on dynamic contact angle. They found that the prediction of wetting kinetics based on the assumption that the surface is ideally smooth is not accurately representing the actual wetting behaviour.
|
(a) Substrate 1 (b) Substrate 2
|
Figure 3. Contact angle variation with time for the two substrates with glycerine/water set 1 (GWS1) and set 2 (GWS2), and with silicone oil set 1 (SOS1) and set 2 (SOS2)
Effect of Drop Base Area
The drop base area is an important geometrical dimension that can be simultaneously obtained along with other parameters (contact angle, drop height and drop volume) from the drop spreading on a surface. The results in terms of drop base area for the two coated paper substrates are shown in Figures 4 (a and b), respectively. The curves for GWS1 and GWS2 and SOS2 are found to be identical on both substrates in which the drop wetted areas reaches maxima at close time periods beyond which it reaches equilibrium and remains almost constant with time prolonging. This going along with the wetting behaviour generally observed.
On contrary, the area wetted in substrate 1 by SOS1 increases drastically until it reaches a maximum value in about 2 seconds and then starts to retract as time goes on as shown in Fig. 4a. Similar wetting behaviour was recoded with SOS1 on substrate 2 (Fig. 4b), but with prolonged wetting time (4 seconds) to achieve maximum wetting area.
|
(a) Substrate 1 |
|
(b) Substrate 2 |
|
glycerine/water: set 1 - GWS1; set 2 - GWS2; silicone oil: set 1 - SOS1; set 2 - SOS2 |
Figure 4. Wetted Drop Base Area variation with time for the two substrates
This obsolete behaviour of SOS1 on both substrates was not observed in the contact angle or drop height variation with time. This suggests that there may be strong interactions (e.g. absorption, diffusion or reaction) between the substrates and the silicone oil sample. Another possibility to explain this behaviour is by considering ‘autophobing’ phenomena as SOS1 has low surface tension lowering the surface energy density in a way that makes the solution no longer wets the surface. The delay in reaching a maximum wetting area on substrate 2 may be attributed to its higher roughness as discussed before.
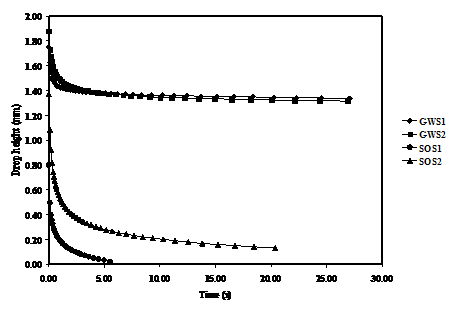
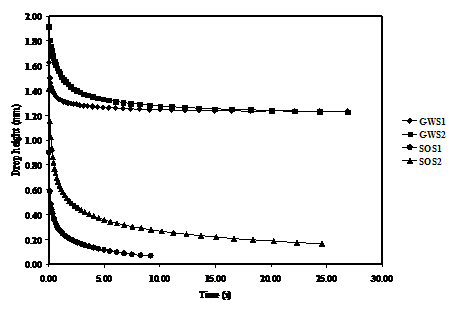
Effect of Drop Height
Variation of the drop height with time was investigated for the two substrates with GWS1, GWS2, SOS1 and SOS2 solutions as shown in Figure 5a and b, respectively.
|
(a) Substrate 1 |
|
(b) Substrate 2 |
|
glycerine/water: set 1 - GWS1; set 2 - GWS2; silicone oil: set 1 - SOS1; set 2 - SOS2 |
Figure 5. Drop Height variation with time for the two substrates
The results in Fig. 5a shows that when the drop is initially placed on the substrate, the drop height with GWS1 and GWS2 solutions decrease rapidly and reaches an equilibrium within 3 seconds then remains almost constant thereafter. Similarly, SOS1 and SOS2 solution caused the drop height to decrease faster than the corresponding GWS1 and GWS2 before reaching equilibrium. These results go along with the observations related to other parameters i.e. surface tension, viscosity and surface roughness investigated in correlation with contact angle discussed earlier.
Considering the wetting behaviour of GWS1 and GWS2 on the two substrates, it can be seen that the difference between the two curves is insignificant despite having different viscosities. On the other hand, comparing wetting curves for SOS1 and SOS2 on the two substrates reveals identical wetting trends with both liquids attaining longer wetting time in substrate 2 as compared to substrate 1. These results can be mainly attributed to the variation in roughness.
Conclusions
Spreading behaviour of liquids with different surface tensions is studied over coated paper substrates. The spreading behaviour is investigated in terms of contact angle and wetted drop base area and the drop height. Based on the findings of this study, the following conclusions can be drawn:
1. The wetting behaviour is strongly dependent upon the physical properties of the liquids (surface tension and viscosity) and roughness of the surfaces.
2. The contact angles increase with increasing the viscosity of liquids. Higher viscosity liquids tend to produce low wettability caused by higher viscous dissipation that reduces the rate of spreading. This coincides with a decrease in the wetted drop base area and an increase in the drop height.
3. The effect of surface tension on the contact angles was found to be more pronounced. Liquids with low surface tension improve the wettability of the paper substrates causing an increase in wetted drop base area accompanied by a decrease in the drop height.
4. Surface roughness is found to affect wetting behaviour to great extent. The measured contact angles records high values with substrates with high surface roughness and vice versa. Thus, smaller wetted base area coupled with larger drop height is obtained.
5. It can be finally concluded that despite being a key factor in determining the wettability, contact angle data alone is not sufficient to fully predict and understand the wetting behaviour of a material. This is due to the complexity arises from the existence of a number of forces affecting the contact line, which vary depending on the applied wetting conditions.
6. The current study reveals that other wetting parameters (wetted area and drop height etc.) have to be taken into consideration to fully understand the liquid spreading behaviour and to set the right combination of parameters to enhance the coating processes and reduce costs.
Acknowledgements
The authors wish to acknowledge the financial support by EPSRC-DTA. The authors are also grateful to Ilford Imaging Switzerland Gmbh (Marly) for providing the substrates.
References
1. Kumar G. and Prabhu K. N., Review of non-reactive and reactive wetting of liquids on surfaces, Advances in Colloid and Interface Science, 133, 61 - 89, 2007.
2. Patankar N. A., On the modeling of hydrophobic contact angles on rough surfaces, Langmuir, 19, 1249, 2003.
3. Patankar N. A., Transition between superhydrophobic states on rough surfaces, Langmuir, 20, 8209, 2004.
4. Sikalo S., Marengo M., Tropea C., and Ganic E. N., Analysis of impact of droplets on horizontal surfaces, Experimental Thermal and Fluid Science, 25, 503 - 510, 2002.
5. Extrand C. W. and Kumagai Y. J., An Experimental Study of Contact Angle Hysteresis, Journal of Colloid and Interface Science, 191 (2), 378 - 383, 1997.
6. Cazabat A. M. and Stuart M. A. C., Dynamics of wetting: Effects of surface roughness, Journal of Physical Chemistry, 90, 5845 - 5849, 1986.
7. Kandlikar S. G. and Steinke M. E., Contact angles and interface behaviour during rapid evaporation of liquid on a heated surface, International Journal of Heat and Mass Transfer, 45 (18), 3771 - 3780, 2002.
8. Ghannam M. T., Spreading behaviour of crude oil over limestone substrate, Journal of Colloid and Interface Science, 262, 435-441, 2003.
9. Zhang Y., Zhang G., and Han F., The spreading and superspreading behaviour of new glucosamide-based trisiloxane surfactants on hydrophobic foliage, Colloids and Surfaces, 276, 100 - 106, 2006.
10. Dutschk V., Sabbatovskiy K. G., Stolz M., Grundke K., and Rudov V. M., Unusual wetting dynamics of aqueous surfactant solutions on polymer surfaces, Journal of Colloid and Interface Science, 267, 456 - 462, 2003.
11. Xiao-dong W., Xiao-feng P., and Bu-xuan W., Effect of Solid Surface Properties on Dynamic Contact Angles, Heat Transfer - Asian Research, 35 (1), 2006.
12. Njobuenwu D. O., Complete wetting of acrylic solid substrate with silicone oil at the centre of the substrate, Australian Journal of Technology, 10 (3), 163 - 168, 2007.
13. Benkreira H. and Khan M. I., Air entrainment in dip coating under reduced air pressures, Chemical Engineering Science, 63, 448 - 459, 2008.
14. NJOBUENWU D. O., Spreading of Trisiloxanes EO3 and EO9 Polyethylene Oxide Surfactant over Hydrophobic Substrates, Leonardo Journal of Sciences, (10), 153 - 164, 2007.
15. Chow T. S., Wetting of Rough Surfaces, J. Phys.: Condens. Matter, 10, 445 - 451, 1998.
16. McHale G. and Newton M. I., Frenkel's method and the dynamic wetting of hetergeneous planar surfaces, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 206, 193 - 201, 2002.
17. Volpe C. D., Maniglio D., Morra M., and Siboni S., The determination of a ‘stable-equilibrium’ contact angle on heterogeneous and rough surfaces, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 206, 47 - 67, 2002.