Topochemical Models for the prediction of 5-HT6 binding affinity of 3-ethyl-1H-indoles
Harish DUREJA and A.K. MADAN
1Faculty of Pharmaceutical Sciences, M. D. University, Rohtak, 124 001, INDIA.
E-mails: harishdureja@gmail.com, madan_ak@yahoo.com
Abstract
Relationship between the topochemical indices and 5-HT6 binding affinity of 3-ethyl-1H-indoles has been investigated. Wienerís topochemical index - a distance-based topochemical descriptor, eccentric connectivity topochemical index and augmented eccentric connectivity topochemical index - both adjacency-cum-distance based topochemical descriptors were used for the present investigation. A dataset comprising of 26 analogues of 3-ethyl-1H-indoles was selected for the present study. The values of Wienerís topochemical index, eccentric connectivity topochemical index and augmented eccentric connectivity topochemical index were computed for each of the 26 analogues using an in-house computer program. Resultant data was analyzed and suitable models were developed after identification of the active ranges. Subsequently, a biological activity was assigned to each compound using these models, which was then compared with the reported 5-HT6 binding affinity. Statistical significance of proposed models was investigated using intercorrelation analysis. Accuracy of prediction of proposed models was found to be 81 - 84%.
Keywords
Topochemical indices; Wienerís topochemical index; Eccentric connectivity topochemical index; Augmented eccentric connectivity topochemical index; 5-HT6 binding affinity; 3-ethyl-1H-indoles.
Introduction
During past decade, topological indices (TIs) have emerged as powerful tools for predicting biological activities of molecules, designing combinatorial libraries and lead identification [1]. A topological descriptor is a numerical descriptor of molecular structure based on certain topological features of the molecular graph, offering an effective way of measuring molecular branching, shape, size and molecular similarity [2]. TIs have several obvious advantages when compared with geometrical, electrostatic and quantum descriptors: they are computed only from information contained in molecular graph, they have a unique value for a particular compound and their calculation requires small computational resources [3]. TIs have been successfully employed in developing a suitable correlation between chemical structure and biological activity by translating chemical structures into numerical descriptors [4, 5]. The topostructural and topochemical indices fall into the category normally grouped together as topological indices. Topostructural indices are topological indices which encode information about the adjacency and distance of atoms in molecular structures, irrespective of the chemical nature of the atoms involved in bonding or factors such as hybridization states and the number of core/valence electrons in individual atoms. Topochemical indices are parameters that quantify information regarding the topology (connectivity of atoms), as well as specific chemical properties of the atoms comprising a molecule [6]. †A limited number of topostructural and topochemical indices have shown their successful applications in structure activity relationships. Some of the these topostructural indices include, Wienerís index [7], Hosoyaís index [8], Randicís molecular connectivity index [9,10], Zagreb group parameters [11], Balabanís index [12], the higher-order connectivity indices, nc, for the paths of length n defined by Kier and Hall [13], eccentric connectivity index [14], Superpendentic index [15], and revised Wiener index [16]. Topochemical indices, which have been successfully employed in structure activity relationship studies, include molecular connectivity topochemical index [17,18], eccentric connectivity topochemical index [19], Weinerís topochemical index [20], Zagreb topochemical indices [21] etc.
The 5-hydroxytryptamine-6 (5-HT6) was one of the additions to the 5-HT receptor family, selective antagonists have recently been developed and potential functional roles are now becoming apparent [22]. The serotonin 5-HT6 receptor, a G-protein-coupled receptor, displays high affinity for antipsychotic, antidepressant, and psychotropic drugs [23]. Various typical and atypical antipsychotic agents and antidepressants have been demonstrated to bind with high affinity at 5-HT6 receptors that these receptors be targeted for the development of novel psychotherapeutic agents [24]. The 5-HT6 receptors appear to regulate cholinergic neurotransmission in the brain, rather than the expected interaction as modulators of dopaminergic transmission. This interaction predicts a possible role for 5-HT6 receptor antagonists in the treatment of learning and memory disorders [25]. 5-HT6 receptors are expressed in brain regions associated with learning and memory, and blockade of their function increases central cholinergic and glutamatergic neurotransmission and enhances cognitive processes. This suggests that the 5-HT6 receptor antagonist-induced enhancement of consolidation involves increased central glutamatergic neurotransmission [26]. The high affinity of a wide range of psychiatric drugs for the 5-HT6 receptor, together with its almost exclusive expression in the CNS, being abundant in limbic and cortical regions, has stimulated significant research interest.22 The 5-HT6 receptor appears to regulate glutamatergic and cholinergic neuronal activity, and increasing evidence suggests that it may be involved in the regulation of cognition, feeding and, possibly, affective state and seizures [27]. Various typical and atypical antipsychotic agents and antidepressants have been demonstrated to bind with high affinity at 5-HT6 receptors that these receptors be targeted for the development of novel psychotherapeutic agents. Evidence also suggest that 5-HT6 receptor might modulate cholinergic transmission and GABA function leading to speculation that 5-HT6 agents could play a role in memory impairment, anxiety, mood-dependent behavior and related disorders [24].
In the present study relationship of Wienerís topochemical index, eccentric connectivity topochemical index and augmented eccentric connectivity topochemical index† with 5-HT6 binding affinity of 3-ethyl-1H-indoles has been investigated.
Material and Method
Calculation of topochemical indices
Wienerís topochemical index (Wc): It is a topochemical version of oldest and most widely used distance based topological index Ė Wienerís index [7] and this modified index takes into consideration the presence as well as relative position of heteroatoms in a hydrogen suppressed molecular structure. Wienerís topochemical index is defined as the sum of the chemical distances between all the pairs of vertices in hydrogen suppressed molecular graph [20], i.e.
![]() †††††††††††††††††††††† †††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (1)
†††††††††††††††††††††† †††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (1)
where ![]() †is the chemical length of the path that
contains the least number of edges between vertex i and j in the
graph G, n is the maximum possible number of i and j.
†is the chemical length of the path that
contains the least number of edges between vertex i and j in the
graph G, n is the maximum possible number of i and j.
Eccentric connectivity topochemical index (xcc): Eccentric connectivity topochemical index is a topochemical version of an adjacency-cum-distance based topological index Ė eccentric connectivity index [14] and this modified index takes into consideration the presence as well as relative position of heteroatom (s) in a hydrogen suppressed molecular structure. Eccentric connectivity topochemical index (xcc) is defined as the summation of the product of chemical eccentricity and the chemical degree of each vertex in the hydrogen suppressed molecular graph having n vertices [19], that is
†††††††††††††††††† xcc 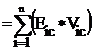 ††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (2)
††††† ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (2)
††††††††††† Where Vic is the chemical degree of vertex i, Eic is the chemical eccentricity of the vertex i and n is the number of the vertices in graph G.
Augmented eccentric connectivity topochemical index (Acxc): It is the topochemical version of the adjacency-cum-distance based augmented eccentric connectivity index28 and this refined index takes into consideration the presence as well as relative position of heteroatom (s) in a hydrogen suppressed molecular structure. It is defined as the summation of the quotients of the product of adjacent vertex chemical degrees and chemical eccentricity of the concerned vertex, for all vertices in the hydrogen suppressed molecular graph [29]. It is expressed as
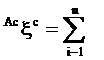 ††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (3)
††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† (3)
where, Mic is the product of chemical degrees of all vertices (vj), adjacent to vertex i, Eic is the chemical eccentricity, and n is the number of vertices in graph G.
Model design and analysis
††††††††††† A dataset comprising of 26 analogues of 3-ethyl-1H-indoles was selected for the present investigation [30]. The basic structure of 3-ethyl-1H-indoles is shown in Figure 1 and the various substituents enlisted in Table 1.
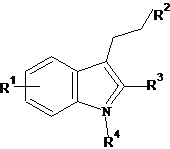
Figure 1. Basic structure of 3-ethyl-1H-indoles
Table 1. Relationships between topochemical indices and 5-HT6 binding affinity
|
Cpd.No |
R1 |
R2 |
R3 |
R4 |
Wc |
xcc |
Acxc |
5-HT6 binding affinity Predicted Reported |
|||
|
1. |
5-OMe |
NH2 |
H |
PhSO2 |
1387.408 |
572.816 |
44.807 |
- |
- |
+ |
- |
|
2. |
5-OMe |
NMe2 |
H |
PhSO2 |
1744.267 |
626.828 |
45.704 |
+ |
+ |
+ |
+ |
|
3. |
5-OMe |
NMe2 |
H |
2-ClC6H4SO2 |
1946.42 |
708.592 |
47.374 |
+ |
+ |
+ |
+ |
|
4. |
5-OMe |
NMe2 |
H |
3-ClC6H4SO2 |
1965.42 |
750.718 |
44.02 |
+ |
+ |
+ |
+ |
|
5. |
5-OMe |
NMe2 |
H |
4-ClC6H4SO2 |
1984.42 |
792.844 |
41.88 |
- |
- |
- |
- |
|
6. |
5-OMe |
NMe2 |
H |
4-MeC6H4SO2 |
1959.945 |
692.33 |
44.129 |
+ |
+ |
+ |
- |
|
7. |
5-OMe |
NMe2 |
H |
4-MeOC6H4SO2 |
2214.277 |
782.421 |
42.586 |
- |
- |
- |
- |
|
8. |
5-OMe |
NMe2 |
H |
2-naphthyl-SO2 |
2628.979 |
841.834 |
45.166 |
- |
- |
+ |
+ |
|
9. |
5-OMe |
NMe2 |
H |
1589.76 |
641.392 |
54.571 |
+ |
+ |
+ |
+ |
|
|
10. |
5-OMe |
NMe2 |
H |
PhCO |
1376.377 |
468.152 |
24.908 |
- |
- |
- |
- |
|
11. |
5-OMe |
pyrrolidinyl |
H |
PhSO2 |
2151.959 |
731.879 |
44.589 |
- |
+ |
+ |
- |
|
12. |
5-OMe |
piperidinyl |
H |
PhSO2 |
2384.305 |
793.769 |
41.701 |
- |
- |
- |
- |
|
13. |
5-OMe |
H |
PhSO2 |
2649 |
876.189 |
38.942 |
- |
- |
- |
- |
|
|
14. |
5-OMe |
morpholinyl |
H |
PhSO2 |
2407.728 |
818.596 |
41.081 |
- |
- |
- |
- |
|
15. |
H |
NMe2 |
H |
PhSO2 |
1407.599 |
567.044 |
44.683 |
+ |
- |
+ |
+ |
|
16. |
H |
NMe2 |
H |
MeSO2 |
700.047 |
356.963 |
36.584 |
- |
- |
- |
- |
|
17. |
5-OBn |
NMe2 |
H |
PhSO2 |
3240.259 |
975.012 |
36.87 |
- |
- |
- |
- |
|
18. |
5-OH |
NMe2 |
H |
PhSO2 |
1561.935 |
593.32 |
45.643 |
+ |
- |
+ |
- |
|
19. |
5-OH |
NMe2 |
H |
PhCO |
1213.052 |
435.619 |
24.437 |
- |
- |
- |
- |
|
20. |
5-OH |
NMe2 |
H |
1111.021 |
386.266 |
24.741 |
- |
- |
- |
- |
|
|
21. |
5-CN |
NMe2 |
H |
PhSO2 |
1738.457 |
618.528 |
46.112 |
+ |
- |
+ |
- |
|
22.A |
5-OMe |
NMe2 |
H |
PhSO2 |
1744.267 |
626.828 |
45.704 |
+ |
+ |
+ |
- |
|
23. |
5-OMe |
NMe2 |
Me |
PhSO2 |
1871.774 |
643.496 |
49.038 |
+ |
+ |
+ |
+ |
|
24. |
5-OMe |
NMe2 |
Me |
H |
536.176 |
262.702 |
20.172 |
- |
- |
- |
- |
|
25. |
H |
NMe2 |
CO2Et |
H |
724.173 |
296.782 |
22.95 |
- |
- |
- |
- |
|
26. |
H |
NMe2 |
B |
H |
2249.414 |
727.646 |
22.235 |
- |
+ |
- |
+ |
+, Active analogue
-, Inactive Analogue
+, Transistional analogue where activity could not be specifically assigned
Aindoline
B3-(3-methoxybenzyl)-1,2,4-oxadiazol-5-yl
The values of the Wienerís topochemical index were computed for each analogue using an in-house computer program. Resultant data was analyzed and suitable model was developed after identification of active range by maximization of the moving average with respect to the active analogues (<35% = inactive, 35-65% = transitional, >65% = active) [31]. Subsequently, each analogue was assigned a biological activity using this model, which was then compared with the reported 5-HT6 binding affinity. 5-HT6 binding affinity was reported [30] quantitatively as Ki at different concentrations. The analogues possessing Ki values of <13 nM were considered to be active and analogues possessing Ki values of >13 nM were considered to be inactive for the purpose of present study. This limit was selected because the drug clozapine, which was use as a control by Russell et al. [30] has a Ki value of 13 nM. Accuracy of prediction of the active and inactive ranges as well as overall degree of prediction of the proposed model was calculated.
A forementioned procedure was similarly adopted for eccentric connectivity topochemical index, xcc and augmented eccentric connectivity topochemical index, Acxc.
The intercorrelation between Wienerís topochemical index, eccentric connectivity topochemical index and augmented eccentric connectivity topochemical index was investigated using the index values of 26 analogues of 3-ethyl-1H-indoles. The degree of correlation was appraised by the correlation coefficient r. Pairs of indices with r>0.97 are considerably highly intercorrelated, those with 0.90<r<0.97 are appreciably correlated, those with 0.50<r<0.89 are weakly correlated and finally the pairs of indices with low r values (<0.50) are not intercorrelated [32]. The results are summarized in Tables 1-3 and Figures 2-5.
Table 2. Topochemical model for 5-HT6 binding affinity.
|
Model Index |
Nature of range |
Index value |
Total Correct |
Percent accuracy |
Average†† Ki (nM)* |
|
Wc |
Lower Inactive Transitional Active Upper Inactive |
<1407.599 1407.599-<1744.267 1744.267-1965.42 >1965.42 |
07 07 04 N.A. 06 04 09 07 |
100.00 N.A. 66.67 77.78 |
174.00 15.80(N.A.) 23.53(8.30) 328.68(421) |
|
xcc |
Lower Inactive Active Upper Inactive |
<626.828 626.828-750.718 >750.718 |
10 09 09 06 07 06 |
90.00 66.67 85.71 |
127.29(141.11) 21.64(7.13) 416.11(483.83) |
|
Acxc |
Inactive Transitional Active |
<44.02 44.02 to <45.704 45.704-54.571 |
13 12 07 N.A. 06 04 |
92.31 N.A. 66.67 |
314.02(340.08) 24.08(N.A.) 21.6 (8.4) |
Values in the brackets indicate average Ki values of correctly predicted analogues of the particular range.
Table 3. Intercorrelation matrix
|
|
Wc |
xcc |
Acxc |
|
Wc |
1 |
0.974 |
0.404 |
|
xcc |
|
1 |
0.537 |
|
Acxc |
|
|
1 |
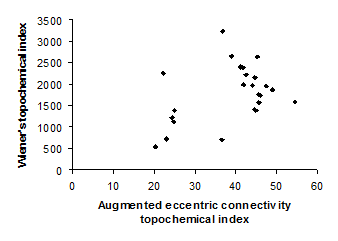
Figure 2. Intercorrelation between augmented eccentric connectivity topochemical index and Wienerís topochemical index
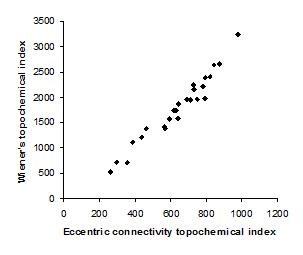
Figure 3. Intercorrelation between eccentric connectivity topochemical index and Wienerís topochemical index.
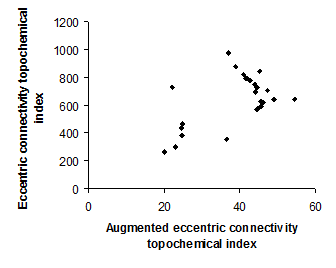
Figure 4. Intercorrelation between augmented eccentric connectivity topochemical index and eccentric connectivity topochemical index.
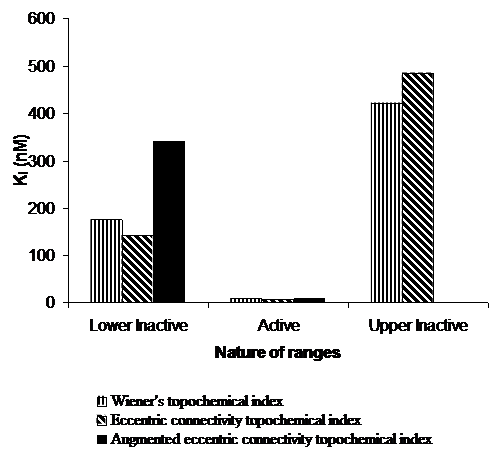
Figure 5. Average Ki (nM) values of correctly predicted analogues of 5-HT6 binding affinity in various ranges of proposed topochemical models.
††††††††††† Results and Discussion
Molecular topology has been demonstrated to be excellent tool for a quick and accurate prediction of physicochemical and biological properties. Matrices from which one can derive a single topological index or a set of them can analytically represent graphs. These indices, whether are well chosen, are a good characterization of molecular structure [33].
††††††††††† Relationship of Wienerís topochemical index - a distance-based topochemical descriptor, eccentric connectivity topochemical index and augmented eccentric connectivity topochemical index Ė both adjacency-cum-distance based topochemical descriptor of 3-ethyl-1H-indoles was studied and suitable models were developed for prediction of 5-HT6 binding affinity. Though all the analogues in the datasets possess varying degree of biological activity but only those analogues having Ki values of <13 nM were considered to be active for the purpose of present study. The methodology used in the present studies aims at the development of suitable models for providing lead molecules through exploitation of the active ranges in the proposed models based on topochemical indices. Proposed models are unique and differ widely from conventional QSAR models. Both systems of modeling have their own advantages and limitations. In the instant case, the modeling system adopted has distinct advantage of identification of narrow active range(s), which may be erroneously skipped during routine regression analysis in conventional QSAR modeling. Since the ultimate goal of modeling is to provide lead structures, therefore, these active ranges can play vital role in lead identification [34].
††††††††††† Retrofit analysis of the data in Tables 1 and 2 reveals following information with regard to Wienerís topochemical index:
ō Out of 22 analogues, 18 (~82 %) were predicted correctly with respect to 5-HT6 binding affinity.
ō The active range had Wienerís topochemical index value from 1744.267 to 1965.42. 67 % analogues in the active range were predicted correctly. The average Ki value was found to be 8.3 nM for the correctly predicted compounds. Extremely low Ki value of 8.3 nM clearly indicates high potency of the active range in the proposed model.
ō The lower inactive range had Wienerís topochemical index values less than 1407.599 and the upper inactive range had Wienerís topochemical index values greater than 1965.42. Activity of ~88% analogues in these inactive ranges was predicted correctly.
ō A transitional range with index values of 1407.599 to <1744.267 was observed. Existence of a transitional range is ideal because it simply indicates gradual change in biological activity.
ō The ratio of average Ki values of active range and lower inactive range was found to be 1:7.4 (1:20.96 for correctly predicted analogues) and ratio of average Ki values of active range and upper inactive range was found to be 1: 13.97 (1: 50.72 for correctly predicted analogues).
††††††††††† Retrofit analysis of the data in Tables 1 and 2 reveals following information with regard to eccentric connectivity topochemical index:
ō Out of 26 analogues, 21 (~81 %) were predicted correctly with respect to 5-HT6 binding affinity.
ō All the compounds in the dataset were classified and there was no transitional range in the proposed model.
ō The active range had eccentric connectivity topochemical index value from 626.828 to 750.718. 67 % analogues in the active range were predicted correctly. The average Ki value was found to be 7.13 nM for the correctly predicted compounds. Extremely low Ki value of 7.13 nM simply indicates high potency of the active range in the proposed model.
ō †The lower inactive range had eccentric connectivity topochemical index values less than 626.828 and the upper inactive range had eccentric connectivity topochemical index values greater than 750.718. Activity of 88% analogues in these inactive ranges was predicted correctly.
ō The ratio of average Ki values of active range and lower inactive range was found to be 1:5.88 (1:19.79 for correctly predicted analogues) and ratio of average Ki values of active range and upper inactive range was found to be 1: 19.23 (1: 67.86 for correctly predicted analogues).
††††††††††† Retrofit analysis of the data in Tables 1 and 2 reveals following information with regard to augmented eccentric connectivity topochemical index:
ō Out of 19 analogues, 16 (84%) were predicted correctly with respect to 5-HT6 binding affinity.
ō The active range had augmented eccentric connectivity topochemical index value from 45.704 to 54.571. 67 % analogues in the active range were predicted correctly. The average Ki value was found to be 8.4 nM for the correctly predicted compounds. Extremely low Ki value of 8.4 nM† indicates high potency of the active range in the proposed model.
ō †The inactive range had augmented eccentric connectivity topochemical index values less than 44.02. Activity of 92% analogues in these inactive ranges was predicted correctly.
ō A transitional range with index values of 44.02 to <45.704 was observed. Existence of a transitional range is ideal because it reveals gradual change in biological activity.
ō The ratio of average Ki values of active range and inactive range was found to be 1:14.54 (1:40.49 for correctly predicted analogues).
Intercorrelation analysis (Table 3) revealed that Wienerís topochemical index is not correlated with augmented eccentric connectivity topochemical index (Figure 2). However, Wienerís topochemical index was highly correlated with eccentric connectivity topochemical index (Figure 3). The augmented eccentric connectivity topochemical index was weakly correlated with eccentric connectivity topochemical index (Figure 4).
Conclusions
Investigations reveal significant correlations of all the three-topochemical indices with 5-HT6 binding affinity of 3-ethyl-1H-indoles. The overall accuracy of prediction varied from ~81% for model based on eccentric connectivity topochemical index to 84% for model based on augmented eccentric connectivity topochemical index. These models offer vast potential for providing lead structure for the development of potent therapeutic agents with regard to high binding affinity for 5-HT6 receptors.
References
1. Estrada E., Patlewicz G., Uriarte E., From molecular graphs to drugs. A review on the use of topological indices in drug design and discovery, Indian J. Chem., 42A, p.1315-1329, 2003.
2. Balaban A. T., Ivanciuc O., Historical development of topological indices, In: Devillers J. and Balaban A. T. (Eds.), Topological Indices And Related Descriptors in QSAR and QSPR, Gordon and Breach Science Publishers, The Netherlands, pp. 21-57, 1999.
3. Ivanciuc O., Taraviras S. L., Cabrol-Bass D., Quasi-orthogonal sets of molecular graph descriptors as a chemical diversity measure, J. Chem. Inf. Cimput. Sci., 40, p. 126-134, 2000.
4. Balaban A. T., Motoc I., Bonchev D., Mekenyan O., Topological indices for structure-activity correlations, Top. Curr. Chem., 114, p. 21-55, 1983.
5. †Basak S. C., Bertelsen S., Grunwald G. D., Application of graph theoretical parameters in quantifying molecular similarity and structure-activity relationships, J. Chem. Inf. Comput. Sci., 34, p. 270-276, 1994.
6. Gute B.D., Basak S.C., Predicting acute toxicity (LC50) of benzene derivatives using theoretical molecular descriptors: a hierarchical QSAR approach, SAR QSAR Environ. Res., 7, p. 117-131, 1997.
7. Wiener H., Correlation of heat of isomerization and difference in heat of vaporization of isomers among paraffin hydrocarbons, J. Am. Chem. Soc., 69, p. 2636-2638, 1947.
8. Hosoya H., Topological index; newly proposed quantity characterizing the topological nature of structure of isomers of saturated hydrocarbons, Bull. Chem. Soc. Jpn., 44, p. 2332-2337, 1971.
9. Randic M., On characterization of molecular branching, J. Am. Chem. Soc., 97, p. 6609-6615, 1975.
10. Randic M., The Connectivity index 25 years after, J. Mol. Graph. Mod., 20, p. 19-35, 2001.
11. Gutman I., Ruscic B., Trinajstic N., Wicox C. F., Graph theory and molecular orbitals. XII. Acyclic polyenes, J. Chem. Phys., 62, p. 3399-3405, 1975.
12. Balaban A. T., Applications of graph theory in chemistry, J. Chem. Inf. Comput. Sci., 25, p. 334-343, 1985.
13. Kier L. B., Hall L. H., Molecular Connectivity in Structure-Activity Analysis, Research Studies Press, Letchworth, UK, 1986.
14. Sharma V., Goswami R., Madan A. K., Eccentric connectivity index: a novel highly discriminating topological descriptor for structure property and structure activity studies, J. Chem. Inf. Comput. Sci., 37, p. 273-282, 1997.
15. Gupta S., Singh M., Madan A. K., Superpendentic index: a novel topological descriptor for prediction of biological activity, J. Chem. Inf. Comput. Sci., 39, p. 272-277, 1999.
16. Randic M., On generalization of Wiener index for cyclic structures, Acta Chim. Slov., 49, p. 483-496, 2002.
17. Goel A., Madan A. K., Structure-activity study on anti-inflammatory pyrazole carboxylic acid hydrazide analogs using molecular connectivity indices, J. Chem. Inf. Comput. Sci., 35, p. 510-514, 1995.
18. Dureja H., Madan A. K., Topochemical models for prediction of cyclin-dependent kinase 2 inhibitory activity of indole-2-ones, J. Mol. Mod., 11, p. 525-531, 2005.
19. Kumar V., Madan A. K., Topological Models for the Prediction of Cyclin-Dependent Kinase 2 Inhibitory Activity of Aminothiazoles, MATCH Commun. Math. Comput. Chem., 51, p. 59 -78, 2004.
20. Bajaj S., Sambi S. S., Madan A. K., Predicting anti-HIV activity of phenethylthiazolethiourea (PETT) analogs: computational approach using Wienerís topochemical index, J. Mol. Struct. (THEOCHEM), 684, p.197-203, 2004.
21. Bajaj S., Sambi S. S., Madan A. K., Prediction of anti-inflammatory activity of N-arylanthranilic acids: computational approach using refined Zagreb indices, Croat. Chem. Acta, 78, p. 165-174, 2005.
22. Woolley M. L., Marsden C. A., Fone K.C. 5-HT6 receptors. Curr. Drug Targets CNS Neurol. Disord., 3, p. 59-79, 2004.
23. Purohit A., Herrick-Davis K., Teitler M., Creation, expression, and characterization of a constitutively active mutant of the human serotonin 5-HT6 receptor. Synapse, 47, p. 218-224, 2003.
24. Tsai Y., Dukat M., Slassi A., Maclean N., Demchyshyn L., Savage J. E., Roth B.L., Hufesein S., Lee M., Glennon R. A., N1-(Benzenesulfonyl)tryptamines as novel 5-HT6 antagonists. Bioorg. Med. Chem. Lett., 10, p. 2295-2299, 2000.
25. Branchek T. A., Blackburn T. P., 5-HT6 receptors as emerging targets for drug discovery. Annu. Rev. Pharmacol. Toxicol., 40, p. 319-334, 2000.
26. King M. V., Sleight A. J., Woolley M. L., Topham I. A., Marsden C. A., Fone K. C., 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidationóan effect sensitive to NMDA receptor antagonism, Neuropharmacology, 47, p. 195-204, 2004.
27. Hirst W. D., Abrahamsen B., Blaney F. E., Calver A. R., Aloj L., Price G. W., Medhurst A. D., Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling, Mol. Pharmacol., 64, p. 1295-1308, 2003.
28. Bajaj S., Sambi S. S., Madan A. K., Model for prediction of anti-HIV activity of 2-pyridinone derivatives using novel topological descriptor, QSAR & Comb. Sci., 25, p. 813-823, 2006.
29. Bajaj S., Study on topochemical descriptors for the prediction of physicochemical and biological properties of molecules, Ph.D. Thesis, Guru Gobind Singh Indraprastha University, India, 2005.
30. Russell M. G. N., Baker R. J., Barden L., Beer M. S., Bristow L., Broughton H. B., Knowles M., McAllister G., Patel S., Castro J. L., N-Arylsulfonylindole derivatives as serotonin 5-HT6 receptor ligands. J. Med. Chem., 44, p. 3881-3895, 2001.
31. Gupta S., Singh M., Madan A. K., Predicting anti-HIV activity: computational approach using novel topological descriptor, J. Comput. Aid. Mol. Des., 15, p. 671-678, 2001.
32. Nikolic S., Kovacevic G., Milicevic A., Trinajstic N., The Zegrab indices 30 years after, Croat. Chem. Acta, 76, p. 113-124, 2003.
33. Garcia-Domenech R., de Julian-Ortiz J. V.,† Duart M. J., Garcia-Torrecillas J.M., Anton-Fos G.M., Rios-Santamarina I., De Gregorio-Alapont C., Galvez J., Search of a Topological Pattern to Evaluate Toxicity of Heterogeneous Compounds, SAR and QSAR in Environ. Res., 12, p. 237-254, 2001.
34. Dureja H., Madan A. K., Models for the prediction of h5-HT2A receptor antagonistic activity of arylindoles: computational approach using topochemical descriptors, J. Mol. Graph. Mod., 25, p. 373-379, 2006.