Biosorption of Heavy Metal Ions from Aqueous Solutions Using a Biomaterial
Innocent OBOH*, Emmanuel ALUYOR, and Thomas AUDU
Department of Chemical Engineering, University of Benin, Benin City, Nigeria.
E-mails: *obohio2009@yahoo.com, eoaluyor@yahoo.com. audutok@uniben.edu
(* Corresponding author)
Abstract
An increase in population initiating rapid industrialization was found to consequently increase the effluents and domestic wastewater into the aquatic ecosystem. Heavy metals are major toxicants found in industrial wastewaters; they may adversely affect the biological treatment of wastewater. Conventional methods for the removal of heavy metals from waste waters are often cost prohibitive hence, there is a need for cheap methods for effluent treatment.
The residual metallic ion concentrations were determined using an Atomic Absorption Spectrophotometer (AAS). The results obtained after contacting for 120 minutes showed that Neem leaves achieved the percent removal of 76.8, 67.5, 58.4 and 41.45 for Cu2+, Ni2+, Zn2+ and Pb2+ ions respectively. The percent removal of Ni2+ ions was 68.75 with an effective dose of 1.0 g of Neem leaves (bioadsorbent). The ability of Neem leaves to absorb metal ions as shown from the results can be used for the development of an efficient, clean and cheap technology for effluent treatment.
Keywords
Neem leaves; Bioadsorbent; Effluents; Heavy metals; Wastewater.
Introduction
The increase in usage of heavy metals in industrial activities has caused the existence of them in waste water. For example lead and cadmium which the wastewater of industries such as electroplating, plastic and paint manufacturing, mining, metallurgical process, petrochemical process, batteries, paper and pulp contains them [1, 2].
The inadequacy of our conventional methods of river dumping was further exposed by the death of fishes and even deforestation of nearby trees on the shore, affecting also, human and animal lives. Therefore, the study of the existing effluent disposal methods, facilities, and attitudes is essential in order to make a positive impact on our environmental hygiene.
The discharge of metallic ions in industrial effluent is of great concern because their presence and accumulation have a toxic effect on living species [3]. Industrial wastewater containing metal ions such as nickel, lead, copper, zinc and aluminium are common because their metals are used in a large number of industries such as electroplating, batteries manufacture, mine, metal finishing, brewery, pharmaceutical, and so on. Heavy metals are toxic to aquatic organisms even at very low concentration. Most of these minerals were present in our environment only in minute amounts until recent centuries, when the orientation toward industrialization and production brought about our many technological advances. But technology, like medicine, has its side effects. At present, these toxic metals have polluted our atmosphere, our waters, our soil, and food chain.
In the discharge of metal ions in industrial effluent using bio-adsorption process has been an area of extensive research because of the presence and accumulation of toxic carcinogenic effect on living species. The most common and harmful heavy metals are aluminium, lead, copper, nickel, chromium and zinc. They are stable elements that cannot be metabolized by the body and get passed up in the food chain to human beings. When waste is disposed into the environment, a further long-term hazard is encountered. There are possibly more problems from these metals, which interfere with normal bodily function, than have been considered in most medical circles. Reviewing all of our vitamins and minerals has shown us that most every substance that is useful can be a toxin or poison, as well. Metals are known primarily and almost exclusively for their potential toxicity in the body, though commercially they may have great advantages.
A conventional method for removing metals from industrial effluents includes chemical precipitation, coagulation, solvent extraction, electrolysis, membrane separation, ion – exchange and adsorption. Most of these methods suffer with high capital and regeneration costs of the materials [4]. Therefore, there is currently a need for new, innovative and cost effective methods for the removal of toxic substances from wastewaters. Bio-sorption is an effective and versatile method and can be easily adopted in low cost to remove heavy metals from large amount of industrial wastewaters. Recent studies have shown that heavy metals can be removed using plant materials such an palm pressed fibers and coconut husk [5], water fern Azolla filiculoidis [6], peat moss [7], duck weed Wolffia globosa [8], lignocellulosic substrate extracted from wheat bran [9], Rhizopus nigricans [10], cork and yohimbe bark wastes [11] and leaves of indigenous biomaterials, Tridax procumbens [12]. Apart from the plant based material chemical modification of various adsorbents, phenol formaldehyde cationic matrices [13], polyethylonamide modified wood [14], sulphur containing modified silica gels [12] and commercial activated charcoals also employed [15].
The Neem tree is noted for its drought resistance. Normally it thrives in areas with sub-arid to sub-humid conditions, with an annual rainfall between 400 and 1200 mm. It can grow in regions with an annual rainfall below 400 mm, but in such cases it depends largely on ground water levels. Neem can grow in many different types of soil, but it thrives best on well drained deep and sandy soils. It is a typical tropical to subtropical tree and exists at annual mean temperatures between 21-32°C. It can tolerate high to very high temperatures and does not tolerate temperature below 4°C.
Neem is a life giving tree, especially for the dry coastal, southern districts. It is one of the very few shade-giving trees that thrive in the drought prone areas. The trees are not at all delicate about the water quality and thrive on the merest trickle of water, whatever the quality. In Tamil Nadu it is very common to see neem trees used for shade lining the streets or in most people's back yards. In very dry areas like Sivakasi, the trees are planted in large tracts of land, in whose shade fireworks factories function.
The aim of this work is to study the removal of toxic heavy metal ions by Neem leaves from synthetic waste water and to offer this biosorbent as local replacement for existing commercial adsorbent materials [16].
Material and Method
Preparation of Neem leaves
The Neem leaves were dried for a period of three days. The Neem leaves were cleaned with distilled water and dried at room temperature. The leaves were grounded with the grinding mill. The ground Neem leaves was sieved and was of particle size 0.25 to 0.5mm. This was to allow for shorter diffusion path, thus allowing the adsorbate (Neem leaves) to penetrate deeper into the effluent more quickly, resulting in a higher rate of adsorption [17].
Preparation of Synthetic Wastewater
The initial concentration used was 5.00mg/l for Copper, 4.00mg/l for Nickel, 20.00mg/l for Lead and 2.50mg/l for Zinc, and the contacting time was varied from 20 to 120 minutes.
A stock solution of Nickel, Lead, Copper, Zinc and Aluminium was prepared in distilled water with Nickel (II) Sulphate, Lead (II) Nitrate, Zinc (II) Sulphate, and Copper (II) Sulphate. All working solutions of varying concentrations were obtained by diluting the stock solution with distilled water. The pH of the effluent was adjusted to a pH of 5 to prevent hydrolysis. The concentration of metal ions in effluent was analyzed by Atomic Absorption Spectrophotometer. For quality control purpose, the diluted water were digested and analyzed with every sample group to track any possible contamination source. A duplicate analyzed for every sample to track experimental error and show capability of reproducing results [18].
Adsorption Experiment
The experiments were carried out in the batch mode for the measurement of adsorption capabilities. The bottles with 500ml capacity were filled with 50ml of the synthetic wastewater, and 1g of Neem leaves (ground). The bottles were shaken for a predetermined period at room temperature in a reciprocating shaker for 2 hours at 300 rpm. The separation of the adsorbents and solutions was carried out by filtration with Whatman filter paper No. 42 and the filtrate stored in sample cans in a refrigerator prior to analysis. The residual metallic ion concentrations were also determined using an Atomic Absorption Spectrophotometer (AAS).
Results and Discussions
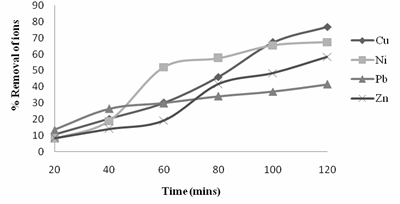
Figure 1. Variation of % removal of ions with Time
Figure 1, showed the percentage removal of the various metal ions by the Neem leaves adsorbent. For all the metal ions present in the synthetic wastewater, there was a progression in the percentage removal of metal ions present in the synthetic wastewater with time. From the result of the adsorption experiment Cu2+ ions had the highest percent removal of 76.8 at the end of 120 minutes, followed by Ni2+ ions, Zn2+ ions and Pb2+ ions with 67.5, 58.4 and 41.45 respectively. For Neem leaves, there was a progression in the rate of adsorption but it was not linear at any time. Also, from figure 1, it was observed that with increase in time, the adsorption rate of the Neem leaves increased. It was also observed that the rate of adsorption increased significantly for some of the metal ions present in the synthetic wastewater between 80 – 100 minutes of contact time. This result is important, as equilibrium time is one of the important parameters for an economical wastewater treatment system.
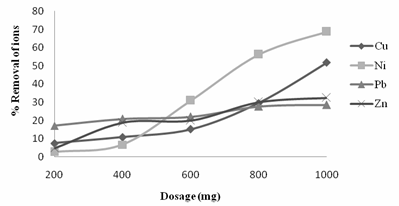
Figure 2. Variation of % removal of ions with Dosage
Figure 2, showed that, the adsorbent dose of 1.0g there was an increase in the adsorption rate. The larger the surface area, the larger the amount of metal ion adsorbed. This appears to be due to the increase in the available binding sites in the biomass for the complexation of the heavy metals [19]. This would probably explain the high percent removal of the heavy metals. The Neem leaves were able to achieve the percent removal of 68.75, 51.8, 32.4, and 28.5 for Ni2+, Cu2+ , Zn2+ and Pb2+ ions respectively.
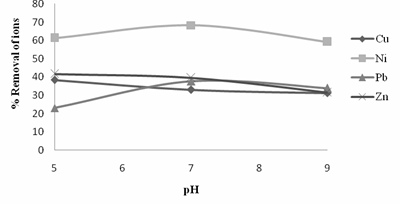
Figure 3. Variation of % removal of ions with pH
Figure 3, showed that Neem leaves had a decrease in the adsorption rate for Cu2+ and Zn2+ ions and an increase in the adsorption rate for Pb2+ and Ni2+ ions when the pH of the synthetic waste water was between the value of 5 and 7. When alkalinity increased that is from pH value of 7 to 9 there was a further decrease in the rate of adsorption by Neem leaves for Cu2+ , Zn2+, Pb2+ and Ni2+ ions in the synthetic waste water . From the results obtained from the adsorption experiment it can be seen that the highest rate of adsorption by Neem leaves was 68.25% removal for Ni ions in the synthetic waste water at pH value of 7. With the increase in pH from 5 to 9, the degree of protonation of the adsorbent functional group decreased gradually and hence removal was decreased. A close relationship between the surface basicity of the adsorbents and the anions is evident. This is similar to the findings of others, where the interaction between oxygen-free Lewis basic sites and the free electrons of the anions, as well as the electrostatic interactions between the anions and the protonated sites of the adsorbent are the main adsorption mechanism [20, 21, 22].
Conclusions
The removal of metal ions in synthetic wastewater by using biosorption technology was studied. Based on the results, the following conclusions can be drawn. The ground Neem leaves was very effective in removal of Ni2+ ions from the synthetic waste water. Neem leaves are efficient biomaterial for removal of some heavy metals from industrial wastewater. The percent removal of Ni2+ ions was 68.75 with an effective dose of 1.0 g of bioadsorbent (Neem leaves). This process can be effectively used in the heavy metals removal in industrial wastewater.
References
1. Iqbal, M. and Edyvean, R.G.J. Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaete chrysosporium, Minerals Engineering, 17, 217 - 223, 2004.
2. Iqbal, M. and Edyvean, R.G.J. Loofa sponge immobilized fungal biosorbent: Arobust system for cadmium and other dissolved metal removal from aqueous solution, Chemosphere, 61, 510 – 518, 2005.
3. Ko, D. C. K., Porter, J. F. and Mckay, G. Optimized Correlation for the Fixed Bed Adsorption of Metal Ions on Bone Char, Chem. Eng. Sci., 55, 5819 – 5829, 2000.
4. Huang, C. P. and Wu, M. H. Chromium removal by carbon adsorption. J. Water. Pollut. Control Fed., 47, 2437-2445, 1975.
5. Tan, W.T., Ooi, S. T. and Lee, C. K., Removal of Chromium (VI) from solution by coconut huskandpalm pressed fibers. Environ. Technol, 14, 277-282, 1993.
6. Zhao, M. and Duncan, J. R. Batch removal of hexavalent chromium by Azolla filiculoides. Biotech. Appl. Biochem. 26, 179-182, 1997.
7. Gosset, T., Traneart, J. L. and Therenot, D. R., Batch metal removal by peat: Kinetics and thermodynamics. Wat. Res. 20, 21-26, 1986
8. Upatham, E.S., Boonyapookana, B., Kriatracjie. M., Pokethitiyook, P. and Parkpoomkamol, K. Biosorption of cadmium and chromium in duck weed Wolffia globosa. Inter. J. Phyto. 4(2), 73-86,2002.
9. Dupont, L., Bouanda, J., Dumoneeau, J. and Applincourt, M. Metal ions binding onto a lignocellulosic substrate extracted from wheat bran: a nica donnan approach. J. Colloid Int. Sci, 263, 35-41, 2003.
10. Bai, R. S. and Abraham, T. E. Biosorption of Cr (VI) from aqueous solution by Rhizopus nigricans. Biores. Technol.. 79, 73-81, 2001.
11. Villaescusa, I., Martinez, M. and Miralles, N. Heavy metal uptake from aqueous solution by cork and yohimbe bark wastes. J. Chem. Technol. Biotechnol.. 74, 812-816, 2000.
12. Freeland, G. N., Hoskinson, R. N. and Mayfield, R. J. Adsorption of mercury from aqueous solutions by polyethylenamine - modified wood fibers. Environ. Sci. Technol.. 8, 953-956, 1974.
13. Singanan, M., Vinodhini, S. andAlemayehu, A. Phytoremediation of heavy metals from industrial waste waters by using indigenous biomaterials. Indian. J. Environ. Prot. 26(5), 385-391, 2006.
14. Swamiappan, N. and Krishnamoorthy, S. Phenol-formaldehyde cationic matrices substitutes by bagasse – Charcoal. Res. Ind.. 29, 293-297, 1984.
15. Verwilghen, C., Guilet, R., Deydier, E., Menu, M. J. and Dartiguenave,Y. Lead and Cadmium uptake by sulphur-containing modified silica gels. Environ. Chem. Lett. 2, 15-19, 2004.
16. Http://en.wikipedia.org/wiki/Neem. (last modified on 1 March 2009, at 11:39.)
17. Adeyinka, A., Liang, H. and Tina, G. “Removal of Metal Ion form Waste Water with Natural Waste” School of Engineering and Technology. 1-8. 33. Ibid 4, 2007.
18. Marshall, W. E. and Champagne, T.E. Agricultural Byproducts as Adsorbents for Metal Ions in Laboratory Prepared Solutions and in Manufacturing Wastewater, Journal of Environmental Science sand Health, Part A: Environmental Science and Engineering. Vol. 30, No. 2, 241 – 261, 1995.
19. Gong, R., Ding, Y., Liu, H., Chen, Q. and Liu, Z. Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere.58(1): 125-130, 2005
20. Leon, Y., Leon, C.A. Solar, J.M. Calemma, V. and Radovic, L.R. Evidence for the protonation of basal plane sites on carbon. Carbon.30. 797–811, 1992.
21. Radovic L.R., Silva, I.F. , Ume, J.I., Menendez, J.A., Leon, C.A., Leon, Y and Scaroni, A.W. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon, 35. 1339-48, 1997.
22. Faria, P.C.C., Orfao, J.J.M. and Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Research, 38. 2043-2052, 2004.