Corrosion Inhibition of AISI/SAE Steel in a Marine Environment
Fatai Olufemi ARAMIDE
Metallurgical and Materials Engineering Dept., Federal University of Technology, Akure, Ondo State, Nigeria.
E-mail: fat2003net@yahoo.com
Abstract
Effect of Sodium nitrite as a corrosion inhibitor of mild steel in sea water was investigated, using the conventional weight loss method. Different percentages of sodium nitrite were used from 0% to 10% in sea water. Samples of mild steel were exposed to these corrosive media and the weight loss was calculated at intervals of 120 hours, 168 hours, 208 hours, 256 hours, 304 hours and 352 hours. It was observed that corrosion rate increases with time of exposure to the corrosive medium (inhibited or non-inhibited) and that sodium nitrite can be used to retards the corrosion rate of mild steel if the appropriate concentration is used in sea water. It was concluded that the optimum percentage of sodium nitrate in sea water that gives the optimum corrosion inhibition of mild steel is 4%.
Keyword
Corrosion inhibitor; Marine environment; Mild steel; Corrosive media.
Introduction
The deterioration of facilities by corrosion is a major problem in Construction Company, oil and gas, ship building and other engineering firms [1-5]. Mild steel is major utilized in most structural shapes such as beams, plates, bars and pipes used both on-shore and off-shore. Considering the severity of the damage caused by corrosion in various engineering field, there is the need to at least retard the corrosion rate, if not to prevent it completely.
There are various methods for prevention of corrosion which basically comprises those protective measures providing separation of metal surfaces from corrosive environments or those which cater for adjustment or altering the environment. These various methods of corrosion prevention include cathodic protection [6, 7], anodic protection [8], coating [9] and the use of corrosion inhibitor [10].
Corrosion inhibitor is a substance which, when added in small concentrations to an environment decrease the corrosion rate considerably. To be fully effective all inhibitors require being present above a certain minimum concentration [10, 11].
Most commonly, corrosion inhibitors are classified as anodic, Cathodic or mixed according to their influence on the electrochemical reaction involving metal, and their environment [12, 13]. Corrosion inhibitors have been in use for several decades and the most familiar examples of their applications are in paints and coatings on metals where nitrate, chromate, phosphate, benzoates, borates and oxides are incorporated, Nitrite is being used as inhibition admixture in concrete reinforcement [14-17].
It is well established that inhibitors function in one or more ways to control corrosion: by adsorption of a thin film onto the surface of a corroding material, by inducing the formation of a thick corrosion product, or by changing the characteristics of the environment resulting in reduced aggressiveness [18]. The focus of this work is to arrive at the optimum concentration of sodium nitrite in sea water to give adequate corrosion to mild steel in marine environment.
Materials and Method
The main material used for this research work are Mild steel (i.e. AISI/SAE 1020) procured from Universal Steels, Ogba Industrial Estate, Ogba, Lagos State, Nigeria. The Spectrometer Analysis of the Mild Steel used is as follow:
Table 1. Spectrometry Analysis Result of the Mild Steel Sample
|
Element |
C |
Si |
Mn |
Cu |
Fe |
|
Weight Percent (%) |
0.203 |
0.152 |
0.627 |
0.204 |
Balance |
The Mild Steel rod was machined down to a sample dimension of 9mm diameter and 10mm length. Thirty-six test samples were made utilized for this test. The cut edges of each of the test samples were smoothened on emery paper, rinsed, dried and weighed on an electronic weighing balance. Each of the test samples is designated and its initial weight is noted.
Six solutions of sodium nitrite in Sea Water was prepared; each having different concentrations of sodium nitrite in sea water. The percentages of the sodium nitrite in the prepared corrosive media are as follow: 0%, 2%, 4%, 6%, 8%, and 10% respectively.
Six test samples were carefully arranged into each of the corrosive media prepared and left for some time (days). After 120 hours of each of the test samples in the corrosive media, one test sample was removed from each corrosive medium at interval, dried weighed to determine the weight loss.
The time intervals used are 120 hours, 168 hours, 208 hours, 256 hours, 304 hours and 352 hours.
Results and Discussion
The weight loss recorded against the percentage Sodium Nitrite in the corrosive media is displayed in Tables 2 to 7 for each time intervals. The weight losses are depicted in Figure 1. The relationship between the weight loss and exposure time is illustrated in Figure 2.
Table 2. Weight modification of steel samples in sodium nitrite and sea water solution after 120 & 168 hours
|
Composition (%) |
120 hours |
168 hours |
||
|
Initial weight (g) |
Final weight (g) |
Initial weight (g) |
Final weight (g) |
|
|
0 |
8.004 |
7.999 |
3.428 |
3.422 |
|
2 |
6.809 |
6.807 |
4.591 |
4.589 |
|
4 |
8.042 |
8.042 |
4.052 |
4.052 |
|
6 |
5.538 |
5.538 |
4.251 |
4.251 |
|
8 |
5.368 |
5.367 |
4.858 |
4.855 |
|
10 |
6.625 |
6.624 |
4.013 |
4.011 |
Table 3. Weight modification of steel samples in sodium nitrite and sea water solution after 216 & 264 hours
|
Composition (%) |
216 hours |
264 hours |
||
|
Initial weight (g) |
Final weight (g) |
Initial weight (g) |
Final weight (g) |
|
|
0 |
5.166 |
5.156 |
4.732 |
4.722 |
|
2 |
4.173 |
4.171 |
4.008 |
4.005 |
|
4 |
4.525 |
4.525 |
5.406 |
5.406 |
|
6 |
4.492 |
4.492 |
4.251 |
4.250 |
|
8 |
4.839 |
4.837 |
5.159 |
5.157 |
|
10 |
4.586 |
4.584 |
5.132 |
5.130 |
Table 4.Weight losses of steel samples in sodium nitrite and sea water solution after 312 & 360 hours
|
Composition (%) |
312 hours |
360 hours |
||
|
Initial weight (g) |
Final weight (g) |
Initial weight (g) |
Final weight (g) |
|
|
0 |
3.410 |
3.399 |
5.306 |
5.289 |
|
2 |
3.856 |
3.851 |
4.539 |
4.532 |
|
4 |
3.677 |
3.676 |
3.180 |
3.179 |
|
6 |
4.252 |
4.250 |
3.953 |
3.951 |
|
8 |
3.222 |
3.220 |
3.372 |
3.369 |
|
10 |
4.098 |
4.095 |
3.028 |
3.025 |
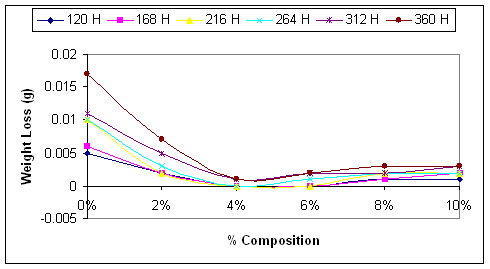
Figure 1. Weight losses of steel samples in sea water solution as affected by the percentage NaNO3 used as Inhibitor
Considering Figure 1 it will be observed that the least corroded samples for the various time intervals are those ones exposed to sea water with 4% sodium nitrite content; the most aggressive corrosive medium is the sea water with no content of sodium nitrite. Looking through the graphs presented in the Figure 1, it is a general trend that the aggressiveness of the sea water reduces with the increase in the percentage sodium nitrite content [19]; this was the case up to 4% sodium nitrite content. There after the aggressiveness of the sea water increase. This is also depicts clearly in Figure 2.
As shown in the figures, the inhibitor does not stop corrosion, but delays its onset thereby leading to decrease in the corrosion rate. Comparing the graphs in Figure 2, it will be seen clearly that the corrosion rate was very high in the case of uninhibited corrosive medium (0% NaNO3), and lowest in the medium with 4% NaNO3. This is in agreement with the findings of [20].
Figure 2 clearly shown that corrosion rate increases with the exposure time of the mild steel to the corrosive media. Even in the medium with 4% NaNO3 where the corrosion rate is the least, the corrosion rate begins to increase from zero level after 264 hours of exposure.
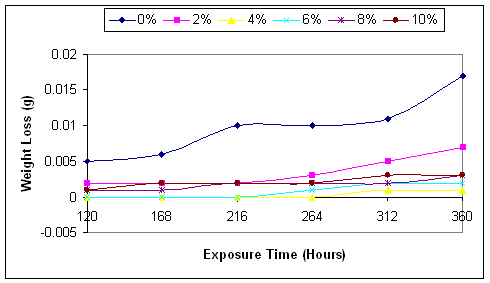
Figure 2.Variations of the weight loss of mild steel in sea water with exposure time
Conclusion
From the discussion so far, it can be concluded that;
1. Mild steel corrodes in marine environment.
2. Corrosion rate increases with time of exposure to the corrosive medium (inhibited or non-inhibited).
3. Sodium nitrite can be used to retards the corrosion rate of mild steel if the appropriate concentration is used in sea water.
4. The optimum percentage composition of Sodium Nitrite required in Sea Water for optimum corrosion inhibiting performance of mild steel is 4%.
References
1. Burubai W., Dagogo G., Comparative Study of Inhibitors on the Corrosion of Mild Steel Reinforcement in Concrete. Agricultural Engineering International: The CIGR Ejornal. 2007, IX , Manuscript BC 06 008.
2. Liu G. Q., Zhu Z. Y., Ke W., Han C. I., Zeng C. L., Corrosion, National Association of Chemical Engineers, 2001, 57(8), p. 730.
3. Ilevbare G. O., Burstein G. T., The Inhibition of Pitting Corrosion of Stainless Steel by Chromate and Molybdate Ions, Corrosion Science, 2003, 45, p. 1545-1569.
4. Munoz A. I., Anton J. G., Nuevalos S. L., Guinon J. L., Herranz V. P., Corrosion studies of Austenitic and Duplex Stainless Steels in Aqueous Lithium Bromide Solution at Different Temperatures, Corrosion Science, 2004, 46, p. 2955-2974.
5. Cui Z. D., Wu S. L., Zhu S. L., Yang X. J., Study on Corrosion Properties of Pipelines in Simulated Produced Water Saturated with Supercritical Co2, Applied Surface Science, 2006, 252, p. 2368-2374.
6. Subasria R., Shinoharaa T., Morib K., Modified TiO2 Coatings for Cathodic Protection Applications, Science and Technology of Advanced Materials 2005, 6, p. 501-507.
7. Kim D. K., Muralidharan S., Ha T. H., Bae J. H., Ha Y. C., Lee H. G., Scantlebury J. D., Electrochemical Studies on the Alternating Current Corrosion of Mild Steel under Cathodic protection Condition in Marine Environments, Electrochimica Acta, 2006, 51, p. 5259-5267.
8. Cecchetto L., Delabouglise D., Petit P., On the Mechanism of the Anodic Protection of Aluminium Alloy AA5182 by Emeraldine base Coatings Evidences of Galvanic Coupling, Electrochimica Acta, 2007, 52, p. 3485-3492.
9. Praveen B. M., Venkatesha T. N., Arthoba Naik Y., Prashantha K., Corrosion Studied of Carbon Nanotubes – Zn Composite Coating, Surface and Coating Technology, 2007, 201, p. 5836-5842.
10. Norman E. Hammer, NACE Glossary of Corrosion Terms, Mat. Pro., 1965, 4(1), p. 79.
11. Nathan C. C., Corrosion Inhibitors, National Association of Corrosion Engineers (NACE), pp. 279, 1973.
12. Dean (Jr.) S. W., Inhibitor Type, Material Performance, 1981, 20(11), p. 47-51.
13. Ramachandran V. S., In: Concrete Admixtures Handbook:Properties, Science and Technology Park Ridge, NJ USA: Noyes Publications, pp. 540-545, 1984.
14. Konno H., Obayashi S. K., Takahash H., Nagayama M., The Hydration of Barrier Oxide Films on Ions. Corrosion Science, 1982, 10, pp. 913-923.
15. Isaacs H. S., Virtanen S., Ryan M. P., Schmuki P., Oblonsky L. J., Incorporation of Cr in the Passive Film on Fe from Chromate Solutions, Electrochimica Acta, 2002, 47, p. 3127-3130.
16. Montes P., Bremner T. W., Lister D. H., Influence of Calcium Nitrite Inhibitor and Crack Width on Corrosion of Steel in High Performance Concrete Subjected to a Simulated Marine Environment, Cement & Concrete Composites, 2004, 26, p. 243-253.
17. Sideris K. K., Savva A. E., Durability of Mixtures Containing Calcium Nitrite Based Corrosion Inhibitor, Cement & Concrete Composites, 2005, 27, p. 277-287.
18. Hackerrman N., In: Fundamentals of Inhibitors, NACE Basic Corrosion Course, Houston, TX: NACE, 1965.
19. Trethewey K. R., Chamberlain J., Corrosion for Science and Engineering, 2nd Edition, Edinburgh Gate Harlow, Essex CM20 2JE England, 1995.
20. Fadayomi J., Corrosion-Inhibitors, Concrete, 1997, 31(8), p. 21-22.