Production and Testing of Coconut Oil Biodiesel Fuel and its Blend
Oguntola J ALAMU1*, Opeoluwa DEHINBO2 and Adedoyin M SULAIMAN2
1Department of Mechanical Engineering, Osun State University, Osogbo, Nigeria
2Department of Mechanical Engineering, Olabisi Onabanjo University, Ibogun, Nigeria
E-mails: tolasum@yahoo.com, teaser_oops@yahoo.com
* Corresponding author
Abstract
Many researchers have successfully worked on generating energy from different alternative sources including solar and biological sources such as the conversion of trapped energy from sunlight to electricity and conversion of some renewable agricultural products to fuel. This work considers the use of coconut oil for the production of alternative renewable and environmental friendly biodiesel fuel as an alternative to conventional diesel fuel. Test quantities of coconut oil biodiesel were produced through transesterification reaction using 100g coconut oil, 20.0% ethanol (wt% coconut oil), 0.8% potassium hydroxide catalyst at 65°C reaction temperature and 120 min. reaction time. The experiment was carried out three times and average results evaluated. Low yield of the biodiesel (10.4%) was obtained. The coconut oil biodiesel produced was subsequently blended with petroleum diesel and characterized as alternative diesel fuel through some ASTM standard fuel tests. The products were further evaluated by comparing specific gravity and viscosity of the biodiesel blend, the raw coconut oil and conventional petroleum diesel.
Keywords
Coconut oil; Biodiesel; Blend; Petroleum diesel; Specific gravity; Viscosity.
Introduction
Fuel and energy crisis and the concern of the society for the depleting world’s non-renewable energy resources led to a renewed interest in the quest for alternative fuels. One of the most promising alternatives fuel is the vegetable oils and their derivatives. The first use of vegetable oil in a compression ignition engine was first demonstrated through Rudolph Diesel who used peanut oil in his diesel engine. The use of oils from coconut, soy bean, sunflower, safflower, peanut, linseed, rape seed and palm oil amongst others have been attempted. The long term use of vegetable oils led to injector coking and the thickening of crankcase oil which resulted in piston ring sticking. Therefore, vegetable oils are not used in diesel engines because of endurance issues [1,2].
To overcome this problem, various modifications of vegetable oils have been employed such as transesterification, micro-emulsion formation and the use of viscosity reducers. Among these, transesterification was considered as the most suitable modification because technical properties of esters are nearly similar to diesel. Through transesterification, these vegetable oils are converted to the alkyl esters of the fatty acids present in the vegetable oil [3-5]. These esters are commonly referred to as biodiesel. Biodiesel is an alternative fuel that is renewable in the sense that its primary feedstock has a sustainable source. Some other feedstocks that can be converted to biodiesel are waste restaurant grease and animal fat [6, 7]. These sources are less expensive than vegetable oil.
In view of the current instability in oil prices, biodiesel stands as an attractive source of alternative energy. By adopting and increasing the use of biodiesel, Nigeria will also be free from her over-dependence on crude oil reserves [8]. Besides, conventional fossil fuel has been reported as being finite. While it is worthy to note that biodiesel will not completely displace petroleum diesel, biodiesel has its place as an alternative fuel and can be a source of lubricity as an additive to diesel fuel. The emissions produced from biodiesel are cleaner compared to petroleum-based diesel fuel. Particulate emissions, soot, and carbon monoxide are lower since biodiesel is an oxygenated fuel. However, emissions of oxides of nitrogen (NOX) are higher when biodiesel is used [9]. The cause of the rise in NOX is unknown and is being studied.
One particular problem of biodiesel is its cold flow properties. Neat biodiesel such as methyl soyate has a pour point (i.e. the lowest temperature at which the fuel is pourable) of -3°C [1]. In colder climates, crystallization can occur, which leads to the plugging of fuel filters and lines. Typically, taking U.S as a case study, biodiesel is blended with diesel fuel. A B20 blend would be 20% biodiesel in diesel fuel [10]. Such a blend would have better cold flow properties compared to neat biodiesel. This work is therefore aimed at producing biodiesel from ethyl esters of coconut oil and comparing some properties of the produced biodiesel with ASTM standards
Material and Method
Transesterification Process
Coconut oil like any other vegetable oils and animal fats are triglycerides, inherently containing glycerine. The biodiesel process (transesterification) turns the oils into esters, separating out the glycerine from the main product (biodiesel). The glycerine sinks to the bottom and the biodiesel floats on top and can be decanted off. The process is called transesterification, which substitutes alcohol for the glycerine in a chemical reaction, using a catalyst.

Figure 1. Transesterification chemistry for ethyl ester (biodiesel) production
Experimental Material
In the Laboratory scale production of coconut oil biodiesel, the following materials were used; 1 litre of coconut oil, 200 ml of ethanol 99+% pure, potassium hydroxide (KOH), blender, scales accurate to 0.1 grams, measuring beakers for ethanol and oil, translucent plastic container with bung and screw-on cap, funnels, bottle for settling and washing, duct tape and thermometer.
The major feedstock source used in this work is coconut oil, locally produced in Nigeria. It was purchased at the local market in Ayetoro, Ogun State, Nigeria. By the stoichiometric equation of the process, 1 mol of coconut oil is required to react with 3 moles of ethanol to produce 3 moles of the biodiesel and 1 mole of glycerol [11]. 100g coconut oil was used for the transesterification process. Reaction temperature for the process must be below the boiling point of alcohol (ethanol, 78°C) used [12]; therefore, a reaction temperature of 65°C was selected. Different researchers have reported different reaction times for transesterification process as well as the entire biodiesel production process. The reported reaction time ranges from less than 30 minutes to more than 120 minutes [13]. Reaction time of 120 minutes was therefore selected.
Most researchers have used 0.1 to 1.2 % (by weight of oil) of catalyst for biodiesel production [13,14]. 0.8% KOH (by weight of coconut oil) concentration was therefore selected while 20% ethanol was used. KOH used was manufactured by Aldrich Chemicals Co. Ltd, England.
Potasium ethoxide Production and Transesterification Reaction
The method of laboratory scale biodiesel production used by Chitra et al (2005) [13] as well as Alamu (2007) [15] is adopted in this work. 20.0g of ethanol was measured and poured into a plastic container through a funnel. 0.8g of KOH was carefully added to the plastic container through another funnel and the container was secured tightly with the bung and the screw-on cap. The container was swirled round thoroughly for about 2 minutes until the KOH completely dissolved in the ethanol, forming potassium ethoxide.
100.0g of coconut oil was measured out in a beaker, pre-heated to 65°C and poured into the blender. The prepared potassium ethoxide from the plastic container was carefully poured into the coconut oil. The blender lid was secured tightly and switch on. The mixture was left to blend for the 120 minutes at moderate speed before the blender was switched off at lowest possible agitation by the blender.
Settling and Washing
The mixture was poured from the blender into a bottle for settling and the lid was screwed on tightly. The reaction mixture was allowed to stand overnight to allow phase separation occurred by gravity settling. The Coconut biodiesel at the top was later carefully decanted leaving the glycerol at the base. Thorough washing of the biodiesel was carried as detailed out in some literatures [8,13]. The process flow chart for the biodiesel production from coconut oil is as shown in Fig.2.
The experiment was repeated three times and average experimental parameters recorded. Specific gravity and viscosity measurements of the virgin coconut oil and a petroleum diesel blend of the ethyl ester produced were made following ASTM standards D1298 and D445 respectively.
Measurable Results and Evaluation Method
Test quantities of raw coconut oil, conventional petroleum diesel and the coconut oil biodiesel/petroleum diesel blend (B10) were subjected to various tests. This was carried out with the facilities available at the Federal Institute of Industrial Research, Oshodi, Nigeria.
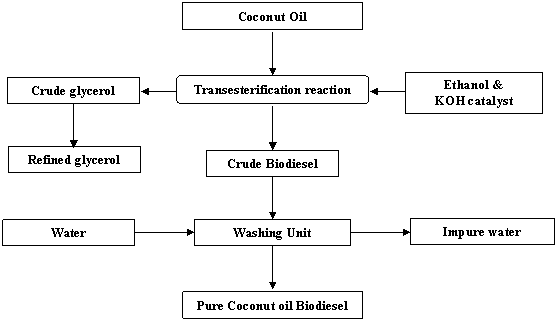
Figure 2. Process flow chart for biodiesel production from coconut oil
Specific Gravity
The specific gravity is a relative measure of the density of a substance. It is defined as the ratio of the density of the substance, ρ, to a reference density, ρref. The most common reference density used in the measurement of specific gravity is the density of water at 4°C, which corresponds to a reference density of 1 g/cc. The specific gravity of the three items was measured using hydrometer.
Specific gravity has been described as one of the most basic and most important properties of fuel and has also been reported to be connected with fuel storage and transportation [8,16].
Specific gravity was determined using equation (1).
![]() (1)
(1)
where: w = weight of specific gravity bottle; w3 = weight of specific gravity bottle + sample; and w4 = weight of specific gravity bottle + water
Viscosity
Viscosity refers to the thickness of the oil, and is determined by measuring the amount of time taken for a given measure of oil to pass through an orifice of a specified size. Viscosity measurement were taken for the three fuel samples at Federal Institute of Industrial Research (FIIRO), Lagos, Nigeria.
Results
Laboratory scale quantities of coconut oil biodiesel produced through transesterification process gave results presented in Table 1. The Table also shows the reaction conditions for the production process. Table 2 shows the fatty acid profile of coconut oil while measured physical/fuel characteristics of coconut oil and the biodiesel blend are as presented in Table 3.
Table 1. Results for the transesterification process
|
Experimental Conditions |
1st Run |
2nd Run |
3rd Run |
Average |
|
Reaction temperature (°C) |
65 |
65 |
65 |
65 |
|
Reaction time (min.) |
120 |
120 |
120 |
120 |
|
Coconut oil quantity (g) |
100 |
100 |
100 |
100 |
|
Ethanol quantity (g) |
20.00 |
20.00 |
20.00 |
20.00 |
|
KOH concentration* |
0.80 |
0.80 |
0.80 |
0.80 |
|
Coconut oil biodiesel obtained (g) |
10.30 |
10.40 |
10.50 |
10.40 |
|
Glycerol obtained (g) |
67.60 |
67.20 |
67.40 |
67.40 |
|
Mass lost (g) |
22.10 |
22.40 |
22.10 |
22.20 |
|
Coconut oil biodiesel yield (%) |
10.30 |
10.40 |
10.50 |
10.40 |
*by weight of 100g coconut oil
Table 2. Fatty acid profile of coconut oil
|
Carbon chain |
Typical values (%) |
Fatty acid |
Method |
|
C6 |
0.2% |
Caprolic acid |
Gas Chromatography (CRL 10.004) |
|
C8 |
4.8% |
Caprylic acid |
|
|
C10 |
4.8% |
Capric acid |
|
|
C12 |
54.5% |
Lauric acid |
|
|
C14 |
18.8% |
Myristic acid |
|
|
C16 |
8.3% |
Palmitic acid |
|
|
C18:0 |
2.8% |
Stearic acid |
|
|
C18:1 |
5.0% |
Oleic acid |
|
|
C18:2N6 |
.8% |
Linoleic acid |
Table 3. Measured physical/fuel characteristics of coconut oil and the biodiesel blend
|
Sample |
Coconut Oil |
Coconut oil biodiesel blend (B10) |
Petroleum (No.2) diesel* |
|
Viscosity at room temperature (mm2/s) |
43.3 |
3.03 |
2.847 |
|
Specific gravity at room temperature |
0.9134 |
0.8305 |
0.853 |
|
*Alamu et. al., 2007 |
|||
A comparison of some physical characteristics of coconut oil, B10 coconut oil biodiesel blend and petroleum diesel is as illustrated in Figure 3.
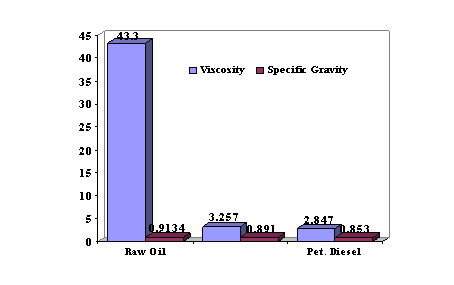
Figure 3. Physical characteristics of coconut oil, B10 coconut oil biodiesel blend and petroleum diesel
Discussion
The yield of the biodiesel from Table 1 was so low in quantity but a substantial amount of volume was needed as a sample so as to effectively investigate its characteristics hence, the need to blend the biodiesel with conventional petroleum diesel through ratio 10 : 90; that is a B10 (10% biodiesel). A typical coconut oil subjected to a fatty acid profile analysis using gas chromatograph, gave the data in Table 2.
From Table 2, it is evident that the carbon chain 12 has the greatest typical value in percentage (54.5%) of the lauric acid amidst others, which describes coconut oil as lauric oil. This in effect tells the nature of the oil used in the production of biodiesel.
Test quantities of raw coconut oil, conventional petroleum diesel and the coconut oil biodiesel B10 blend were subjected to various tests. Some of the important physical characteristics of coconut oil as conducted at the Federal Institute of Industrial Research, Oshodi are as presented in Table 3.
From Table 3, it is observed that the viscosity of the coconut oil is very high (43.3 mm2/s). This is consistent with reported results on vegetable oils. As pointed out in earlier works carried out by Peterson et al., (1990) [1] and Alamu et al., (2007) [8], high viscosities of pure vegetable oils reduces the fuel atomization and increase fuel spray penetration, which would be responsible for high engine deposits and thickening of lubricating oil that cause injection coking and ring stickening of the engine and therefore compromising the efficiency of the engine.
It can also be deduced from Table 3 that the specific gravity of the coconut oil is higher (0.9134) than the conventional petroleum diesel. This indicates that the coconut oil is denser than the No.2 diesel. This is in agreement with reported works of Peterson et al. (1990) [1], Graboski and McCormic (1998) [2] and Yuan et al. (2004) [17] on vegetable oils and fossil diesel fuel.
The transesterification process yielded 10.4g coconut oil biodiesel and 67.4g glycerol, while 22.20g of the total reacting masses could not be accounted for. Such losses have been attributed to some un-reacted alcohol, residual catalyst and emulsion removed during the washing stage of the production process [13]. As a result of the low yield of the coconut biodiesel recorded, a B10 blend was produced. Comparison of the physical characteristics of the coconut oil, the B10 blend and petroleum diesel are made in Table 3.
With the values presented in Table 3, it is observed that the value of the viscosity of the B10 sample is thus far less than that obtained with the raw coconut oil sample. Therefore, the spraying effect that is produced during fuel injection into the combustion chamber of the engine is improved. It is significant from arithmetical calculations that the percentage reduction of viscosity is 92.48% and this indicates a very promising blend that would enhance the cold flow properties of the biodiesel blend.
Also, it is seen from Table 3 that the B10 specific gravity (0.8305) is less than that of the coconut oil (0.9134). It has been reported that, specific gravity has correlations with the cetane number and the heating value of a fuel [8]. Hence, this is an indication that the biodiesel blend is less dense than the coconut oil.
Conclusions
The potential for use of coconut oil for the production of alternative renewable and environmental friendly diesel fuel (biodiesel) was investigated. Test quantities of coconut oil biodiesel were produced through transesterification reaction using 100g coconut oil, 20.0% ethanol (wt% coconut oil), 0.8% potassium hydroxide (KOH) catalyst at 65°C reaction temperature and 120 min. reaction time. The process yielded 10.4% biodiesel. The coconut oil biodiesel produced was subsequently blended with petroleum diesel and characterized as alternative diesel fuel through some ASTM standard fuel tests.
References
1. Peterson C.L., Cruz R.O., Perkings L., Korus R., Auld D.L., Transesterification of vegetable oil for use as diesel fuel: A progress report., ASAE Paper 1990, No. 90-610.
2. Graboski M.S., McCormick R.L., Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sc., 1998, 24, p. 125-164.
3. Al Widyan M.I., Al Shyoukh A.O., Experimental evaluation of the transesterification of waste palm oil into biodiesel, Bioresources Technology, 2002, 85(3), p. 253-256.
4. Lang X., Dalai A.K., Bakhshi N.N., Reaney M.J., Hert P.B., Preparation and characterization of biodiesels from various biooils, Bioresces Technology, 2001, 80, p. 53-62.
5. Ramadhas A.S., Jayaraj S. and Muraleedharan C., Biodiesel production from high FFA rubber seed oil. Fuel, 2005, 84, p. 335-340.
6. Felizardo P., Correira M.J.N., Raposo I., Mendes J.F., Berke-Meier R., Bordado J., Production of biodiesel from waste frying oil., Waste Management, 2006, 26, p. 487-494.
7. Yong W., Ou S., Liu P., Zhang Z., Preparation of biodiesel from waste cooking oil via two-step catalyzed process, Energy Conversion Management., 2007, 48, p. 184-188.
8. Alamu O.J, Waheed M.A., Jekayinfa S.O., Biodiesel Production from Nigerian Palm Kernel Oil: Effect of KOH Concentration on Yield”, Energy for Sustainable Development, 2007, XI(3), p. 77-82.
9. Munack A., Schroder O., Krahl J., Bunger J., Comparison of relevant exhaust gas emissions from biodiesel and fossil diesel fuel. Agricultural Engineering International: the CIGR Journal of Scientific Research and Development. Manuscript EE 01 001 Vol. III, 2001.
10. Ajav E.A., Akingbehin A.O., A study of some fuel properties of local ethanol blended with diesel fuel. Agricultural Engineering International: the CIGR Journal of Scientific Research and Development. Manuscript EE 01 003 Vol. IV, 2002.
11. Kavitha P.L., Studies on transesterified mahua oil as an alternative fuel for diesel engines. M.Sc. thesis: Anna University, Chennal-600 025. India, (2003).
12. Van Gerpen J., Biodiesel processing and production. Fuel Processing Technolngy, 2004, XX, p.1-11.
13. Chitra P., Venkatachalam P., Sampathrajan A., Optimisation of experimental conditions for biodiesel production from alkali-catalysed transesterification of Jatropha curcus oil. Energy for Sustainable Development, 2005, IX(3), p. 13-18.
14. Alcantara R., Amores J., Canoira L., Fidalgo E., Franco M.J., Navarro A. Catalytic production of biodiesel from soybean oil, used frying oil and tallow. Biomass and Bioenergy, 2000, p. 515-527.
15. Alamu O.J. Optimal operating conditions for the production of biodiesel from palm kernel oil. Unpublished Ph.D. thesis: Mechanical Engineering Department. Ladoke Akintola University of Technology, Ogbomoso, Nigeria, 2007.
16. Yuan Y., Hansen A., Zhang Q. The specific gravity of biodiesel fuels and their blends with diesel fuel. Agricultural Engineering International: the CIGR Journal of Scientific Research and Development, 2004, VI, Manuscript EE 04 004.