Development and Characterization of a Carbonated Ginger Drink
Saka Ambali ABDULKAREEM1,*, Habibu UTHMAN2, Abdulfatai JOIMOH3
1Department of Civil and Chemical Engineering, College of Science, Engineering and Technology, University of South Africa, Private Bag X6, Florida 1710, Johannesburg, South Africa
2Membrane Research Unit (MRU), Block L-01, Universiti Teknologi Malaysia (UTM), International Campus, Jalan Semarak, Private Bag 54100 WP, Kuala Lumpur, Malaysia
3Department of Chemical Engineering, Federal University of Technology, PMB 65, Bosso, Minna, Niger State, Nigeria
E-mail: abdulas@unisa.ac.za
*Corresponding Author: Tel: +27114712829; Fax: +27 114713054.
Received: 10 June 2010 / Accepted: 10 May 2011 / Published: 24 June 2011
Abstract
There is widespread of effort in developing countries like Nigeria to develop carbonated drink from the locally source materials. To achieve this aim, ginger has been consider as an alternative material for the production of carbonated drink, this is because ginger and its by-products have a lot of applications in confectionaries, pharmaceuticals and beverages production. Apart from the applications of ginger listed above, ginger is also a very important and highly valuable crop in most part of West African, Japan, and China. In this study, ginger soft drink was developed by a combination of fresh ginger rhizome and Carbon dioxide (CO2). The CO2 gas volume was steadily increased to make three (3) samples B, C, and D, while sample A is the non-carbonated sample. All the samples were characterized according to chemical and microbiological analysis of the final product and values obtained from the analysis of the developed ginger soft drink are within the range of standard values for carbonated drink. Shelf-life analysis was carried out on the final product and it corresponds to the Shelf-life of a standard carbonated drink. Sample D with the highest gas volume of 2.8 cm3 has the best Shelf-life. Results obtained from the various analysis conducted indicates that the production of a carbonated ginger soft drink from fresh ginger rhizome is practically possible, cost effective and has numerous advantages when compared with other available carbonated drinks.
Keywords
Ginger soft drink; Fresh ginger rhizome; Shelf-life; Carbonated drink.
Introduction
Ginger is a very important and highly valuable crop in some countries like Nigeria, Sierra-Leone, India, Japan, and China. This, perhaps is because ginger and its derivations (e.g. ginger oil, ginger powder, ginger syrup or juice and ginger flakes) have a lot of applications which include confectionaries, pharmaceuticals and beverages production [1]. Ginger is composed of water, protein, fat, starch, fiber, ash, volatile oil and resinous matter [2]. Some biologically active components are: asparaginase, borneol, chavicol, citral, cumene, cymene, geraniol, gingerdiorie, gingerol, limonene, myrecene, neral, piriene, shogal and zingerone.
Studies have shown that ginger has pronounced anti-oxidant activity, reduces inflammation and help in arresting narcotic addiction [3] .Ginger significantly inhibits the growth of both gram-positive and gram-negative bacteria. It is a stimulant, when chewed it increases the flow of saliva. When swallowed it acts as a stimulating tonic, increases the secretion of gastric juice, excites alimentary muscular system and dispels gases accumulated in stomach and bowels [4]. Spicy aromatic ginger is advantageous to human body; it is effective for indigestion and also helps in preventing the systems of motion sickness [4].
Thus, demand for ginger and its products at local and international market is so high that it was rated 10th most important commodities at the world trade market level [5]. Recent development in ginger processing shows that it can be processed into ginger soft drink from fresh rhizome [6]. The product was observed to have a Shelf-life of 24 hours. To increase the Shelf-life therefore, there is the need for proper formulation and carbonation.
Carbonated drinks are desired and preferred. They have sharp, unique and refreshing taste. They are non-alcoholic beverages that consist of CO2, water, flavouring and sweet syrup [7]. Carbonated ginger drinks are also found to possess a lot of comparative advantages over other available carbonated drinks, some of which include: improve medicinal values, ease digestion and adsorption of food in human system, boost immune system against infections, clean the cholesterol in the blood veins, warm the body, liberate stagnant fluid in the system, and help to combat morning sickness in pregnant women [4].
The presence of Nigerian ginger in the international market is gradually being noticed [8]. It was asserted that ginger was introduced into Nigeria by a Jamaican [8]. The major producing areas include Kaduna, Nassarawa, Sokoto, Zamfara, Akwa-Ibom, Abia and Lagos States. Of these, Kaduna State is the highest producer of fresh ginger and subsequently has become an attractive market area [8,9]. The first ginger market in Nigeria began in Kwatun and Kachia Local Government Areas of the State. Shipment to international market was mainly to London, and later to United States of America and Canada. Due to the awareness of ginger as foreign exchange earner, many individual and companies now cultivate and process ginger in large quantities [9,10]. However, international standard dictates the pace at which Nigerian ginger can favourably compete with other ginger from other places like Jamaica or India [11,12].
The aim of this study is to produce carbonated ginger soft drink from locally source fresh ginger rhizome and carbon dioxide (CO2), characterize it to determine its suitability as an alternative carbonated drink and present the results obtained from the experiments carried out on produced and characterized carbonated ginger soft drink.
Material and Method
Formulation of Ginger Drink
The sample materials used in this work are fresh ginger rhizome and various food additives such as citric acid, sucrose and sodium benzoate. Fresh ginger was properly sort to remove roots and undesirable particles. It was then washed in clean water weighed and crushed in a pulverizer. Little water was added for easy crushing. 10 dm3 of water was added to every 1 kg of pulverized ginger. Muslin cloth was used for filtration and raffinate discarded. The filtered solution was allowed to stand for 2-3 hours to enable the starch settled (sediment). Mother liquor of the water soluble extract was decanted from the sediment starch. The pure ginger extract was heated to about 70°C to eliminate microorganisms. It was then blended with food additives mentioned above in accordance with standard set by the World Health Organisation (WHO). The resulting solution is a ginger soft drink.
To carbonate the product, it was first pre-chilled in a refrigerator to lower the temperature to about 2-4°C for easy absorption of CO2, which was then added to the ginger with the aid of carbonator. The temperature and pressure of the carbonator gauge varied at three different stages to vary the volume of CO2 in the ginger soft drink represented as sample B, C and D while the sample A is the soft drink that was not carbonated. The product was then bottled and sealed immediately for freshness. The product (carbonated ginger drink) was then analysed to determine the turbidity, ash content, pH, Titratable acidity, brix and microbial count.
Characterization of Ginger Drink
§ Turbidity Determination: Turbidity of the ginger was determined using turbid meter, which was calibrated using an already prepared standard. The ginger extract sample was shaken thoroughly to disperse solid in the sample and poured directly into the turbid meter cell. The turbidity was read directly from the digital scale.
§ Ash Content Determination: A cleaned flat bottom flask silica disc was held for about one minute. It was then transferred to a dessicator to cool down and then weighed. 50cm3 of the ginger sample was taken into the dish and the total weight was obtained. The silica dish and the sample were heated gently on a bursen burner in a fumed cupboard until the smoke ceased. It was then transferred to a muffled furnace heated to about 500°C. The temperature was maintained for about 4hours to ensure that the whole carbon was burnt away. The furnace was switched off and silica dish removed, covered immediately and placed inside a dessicator to cool and then weighed.
§ pH Determination: 50 cm3 of product was put into a beaker of pH meter and the pH was read directly from the digital scale.
§ Determination of Titratable Acidity: A clean burette was filled with 100 cm3 of Sodium hydroxide solution and level of NaOH solution adjusted to zero mark. 50 cm3 of ginger was pipette into a conical flask and two drops of phenolphthalein indicator was added. NaOH solution was titrated against the ginger sample, swirling the conical flask to mix the two solutions and titration was continued until the end point was reached. The volume of alkali- NaOH solution used to neutralize the acid in the ginger sample was recorded.
§ Brix Determination: Rectangular brix equipment was used to measure the brix (sugar content) of the product. A little drop of the product was poured on the space provided on the equipment. The percentage sugar concentration was then viewed from the optical lens provided in the instrument.
§ CO2 Gas Volume Determination: The sample was equilibrated by gently inverting the sample bottle 20 times in 30 seconds and waited for bubbles to settle in the liquid. The sample was placed under the Zahmnagel tester and align crown with the piercing device. The snift valve was closed at the front of the tester. The grasp springs and cross bar was carefully lowered until the piercing needle rested on the crown. The crown was pierced forcing the cross bar down with a firm and rapid motion. The head pressure was released by carefully opening the snift valve, allowing the pressure to drop and close the valve closely. The bottom of the tester pad was held in one hand and the cross bar in the other hand. The tester was shaken vigorously in horizontal motion until the maximum pressure gauge read a constant pressure. The maximum pressure was recorded and the cross bar removed. A thermometer was inserted immediately and the temperature reading recorded. The pressure and temperature readings obtained were converted to gas volume using a volume chart.
§ Microbiological Analysis of the Product (Determination of yeast and mould): 10cm3 of ginger product’s sample was pipette into 90cm3 of nutrient agar solution and mixed thoroughly. 1cm3 of the homogeneous solution was pipette into tubes with potato dextrose agar medium containing tartonic acid and diluted serially i.e., 10-1, 10-2, 10-3 and 10-4 respectively. The sample from the last series was taken and incubated at 37°C for 48 hours after plate counting was carried out.
§ Determination of Mesophilic Bacteria/Total Aerobic Bacterial: 1cm3 of the ginger sample was pipette into a marked petri-dish and mixed thoroughly with the ginger sample. It was incubated at 37°C after which plate counting was carried out with the aid of powerful electronic microscope.
§ Sensory Evaluation: The available sample of ginger soft drink was given to the respondents with a copy of questionnaire to be filled based on the sample with preferred taste, colour, flavour and acceptability.
§ Shelf-life Determination: Within the period of storage the following analysis were carried out: periodic pH determination, periodic titratable acidity determination, periodic changes in microbial count.
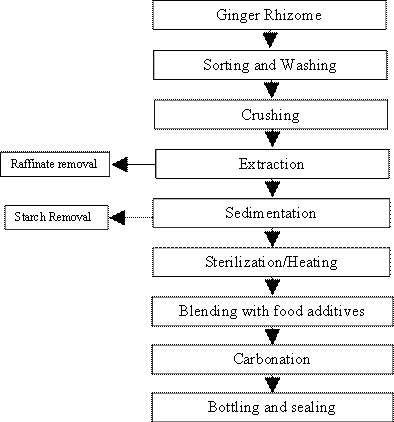
Figure 1. Production processes of carbonated ginger drinks
Results and Discussion
Ginger is a very popular medicinal crop with international recognition. It significantly inhibits the growth of both the gram-positive and gram-negative bacteria [12]. It has been proved that ginger is a potent thromboxane synthesis; it also has therapeutic capabilities in alcohol withdrawal. Ginger is generally processed for international market in form of three primary products i.e. fresh ginger, preserved ginger and dried ginger.
The aim of this article is to present the experiments carried out in the development and characterization of carbonated ginger drink and various results obtained are presented. Figiure1 shows the flow diagram of the production processes of carbonated ginger drinks. While the results of the various analysis conducted on the carbonated ginger drink are summarized in Tables 1-5.
Table 1. Analysis of the pure ginger extract
|
|
Pure Ginger Extract |
Standard |
|
Turbidity (NTU) |
2 |
1 |
|
Ash content (%) |
6 |
13 |
Table 1 shows the experimental result obtained for the analysis of the ginger extract. A turbidity test of 2 NTU was obtained, which is above the standard for all carbonated drink that supposed to be a maximum of 1 NTU. The high turbidity value on any drink to be carbonated is that the absorption of CO2 by the drink may be difficult [6]. For instance, at an extreme value of 3 NTU and above there will be a total reverse of the process i.e., total rejection of CO2 by the drink in the carbonator. The ash content of 6% is within the acceptable range of any raw material to be processed into consumable product.
Table 2. Characterization of carbonated ginger drink
|
Samples |
A |
B |
C |
D |
Standard |
|
pH |
4.20 |
3.40 |
3.10 |
2.80 |
2-4.5 |
|
Titratable (%) |
0.27 |
0.48 |
0.52 |
0.57 |
0.1-2.0 |
|
Gas volume (cm3) |
0.00 |
2.00 |
2.50 |
2.80 |
2 – 4 |
|
Brix (%) |
10.8 |
10.9 |
10.7 |
11.0 |
10-14 |
Result in Table 2 shows the chemical analysis of the final product. The pH value of 4.2 was obtained for the non-carbonated sample A and is the least acidic among all the samples probably because it was not carbonated and contains non-carbonic acid.
The pH value for carbonated samples B, C, and D were found to be 3.40, 3.10 and 2.80 respectively. It was observed that there was a decrease in the pH of the samples from B to D and this could be attributed to the proportion of CO2 added to the samples which in turn affects the concentration of the carbonic acid (H2CO3) present in the drink [6]. Percentage titratable acidity increases from the non-carbonated sample A to the carbonated samples B-D. An increase in gas volume was observed according to the preparation, i.e., 2.0, 2.5 and 2.8 respectively. Hence, high level of turbidity in the drink makes gas volume reading not to correspond to the increase in variation of the temperature and pressure gauge which determines the gas volume content. The % Brix (sugar content) was observed to be approximately the same for all the samples, (10.8, 10.9, 10.7 and 11 for A, B, C and D respectively) irrespective of the degree of carbonation. This was found to be within the range of the standard carbonated drink.
Table 3. Microbiological Analysis of carbonated ginger drink
|
Samples |
A |
B |
C |
D |
Standard |
|
Bacterial count |
14 |
10 |
9 |
9 |
20 |
|
Yeast and Mould |
8 |
7 |
6 |
5 |
10 |
Result in Table 3 shows microbiological analysis of the final product. For the bacteria, yeast and mould count, the non-carbonated sample A has the highest count of 14 and 18 respectively probably because of its low acidic content and a gradual decrease in the bacteria. Yeast and mould count was observed for the carbonated samples B-D. Carbonated sample D has the lowest bacteria; yeast and mould count of 9 and 5 respectively. This could be attributed to high acidic medium which inhibit the growth of micro organisms.
The general observation from the micro-biological analysis shows that as the medium becomes more acidic (pH and titratable evaluation) the concentration of micro-organism continues to reduce.
Table 4. Sensory evaluation
|
Samples |
A |
B |
C |
D |
Total Number of Respondents |
|
Respondent |
2 |
4 |
14 |
5 |
25 |
Result in Table 4 shows the sensory evaluation of the final product. A total number of 25 respondents were recorded for all samples. The non-carbonated sample A has the lowest acceptance number of 2 respondents while carbonated sample C has the highest number of respondents of fourteen (14). This was considered the most acceptable. However, carbonated sample B recorded four (4) respondents and it was observed that the sample has a still taste i.e., tasting like non-carbonated product. This was considered to be so perhaps because of the low carbonic acid concentration present [6] .While sample D with total respondents of five was observed to have sour taste resulting from high concentration of carbonic acid in it.
Results in Table 5 shows the Shelf-life of the final product carried out at every three (3) weeks interval. The general observation from the Table shows that as the pH decreases for each sample, the titratable acidity was increasing. This implies that as the product gets spoilt, its acidic content increases and the product become more viscous probably because of acetic acid contained in fresh ginger rhizomes. The total count shows that non-carbonated sample A has a rapid increase in aerobic count and it recorded sixteen count after 9 weeks of analysis, probably because it was not carbonated and it was presumed to last for only about six months, while the highest microbial count in carbonated sample B, C and D after the same 9 weeks of analysis are 14, 13, and 11 respectively. Only carbonated sample D has slow responses in microbial growth and presumed to be the best among all the samples. It is presumed to last for about 12 months.
Table 5. Shelf-life Analysis of ginger drinks (three weeks Interval determination)
|
Samples |
Weeks |
pH |
Titratable acidity (%) |
Total aerobic count |
|
A |
3 |
4.00 |
0.27 |
13 |
|
6 |
3.60 |
0.29 |
15 |
|
|
9 |
2.90 |
0.50 |
16 |
|
|
B |
3 |
3.00 |
0.48 |
11 |
|
6 |
2.93 |
0.50 |
13 |
|
|
9 |
2.81 |
0.52 |
14 |
|
|
C |
3 |
2.90 |
0.50 |
11 |
|
6 |
2.85 |
0.52 |
12 |
|
|
9 |
2.72 |
0.57 |
13 |
|
|
D |
3 |
2.70 |
0.57 |
10 |
|
6 |
2.70 |
0.57 |
10 |
|
|
9 |
2.75 |
0.58 |
11 |
Therefore, the developed carbonated ginger drink is within the range of Shelf-life of a standard carbonated drink.
Conclusions
In this study, carbonated ginger drink was developed and characterized to determine the suitability of the drink as an alternative carbonated source from locally material. Results obtained revealed that both chemical and microbiological characterization of the ginger drink developed falls within the acceptable standard value and the analysis also reflects that the final products contained no harmful microorganisms or by-product.
It was found that the sample with the highest gas volume also has the best Shelf-life and the non-carbonated sample yields easily to microbial growth. Therefore, this research work clearly shows that the development of a carbonated ginger drink is practically possible and profitable.
References
1. Van-Oss J.F., Material and Technology of Product, London, Longman De Bussy, 1970.
2. David P., The Chemical Analysis of Food, London, Churchill, 1970.
3. Akomas E.C., Oti E., Development for Processing of Nigerian Ginger, Dolf Madi Publication, Nigeria, 1989, p. 93.
5. Cocks L.V., Varrde C., Laboratory Handbook for Oil and Fat, London, Academic Press, 1976.
6. Pigman W., Horton D., The Carbohydrates Chemistry and Biochemistry, London, Pergamon Press, 1971.
7. Howitz W., Method of Chemical Analysis, Association of Official Analytical Chemists (AOAC), 1970.
9. Njuku B.O., Ginger Production in the Tropics, Dolf Madi Publication Nigeria, 1995, 3(4), p. 65-71.