Corrosion Behaviour of Heat - Treated Al-6063/ SiCp Composites Immersed in 5 wt% NaCl Solution
Kenneth ALANEME*
Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, PMB 704, Nigeria
E-mail: kalanemek@yahoo.co.uk
Received: 1 December 2010 / Accepted: 13 May 2011 / Published: 24 June 2011
Abstract
The influence of SiC volume percent and temper conditions (namely, as-cast, solutionized, and artificial age hardening at 180°C and 195°C) on the corrosion behaviour of Al (6063) composites and its monolithic alloy immersed in 5wt% NaCl solution has been investigated. Al (6063) - SiC particulate composites containing 6, 12 and 15 volume percent SiC were produced by premixing the SiC particles with borax additive and then adopting two step stir casting. Mass loss and corrosion rate measurements were utilized as criteria for evaluating the corrosion behaviour of the composites. The results show that the corrosion susceptibility of the Al (6063) - SiCp composites was higher than that of the monolithic alloy, and for most cases the corrosion rate of the composites increased with increase in volume percent of SiC. However, it was discovered that the nature of the passive films formed on the composites was sufficiently stable to reduce significantly the corrosion rate of the composites after 13days of immersion. This trend was observed to be consistent for all heat-treatment conditions utilized.
Keywords
Stir casting; Al (6063); SiCp composite; age - hardening; Corrosion; NaCl solution.
Introduction
Materials design requires a lot of considerations before selections are made, especially when novel techniques are utilized to process known materials variations in material behaviour are to be expected. This will not likely be an exception in the case of the development of Al (6063) matrix composites which is gaining a lot of interest particularly in developing countries [1]. The impetus for this spans from the numerous property advantages that are offered by Al based matrix composites which includes high specific strength and stiffness, improved high temperature properties, controlled thermal expansion coefficient, improved wear and abrasion resistance among others [2-4]. Al (6063) alloy is processed in very large quantities at low cost for mostly building and architectural design works in most developing countries and it is thus quite understandable why attention is focused on Al (6063) alloy based composites. Considerations of mechanical properties of Al (6063) matrix composites have received some attention [1] and the behaviour in acidic environments such as HCl - H2SO4 (typical of the chemical industries) is under investigation [5]. Generally, the corrosion performance of Al-based SiC composites has been a major concern to researchers as efforts made to understand the corrosion behaviour of these composites, yielded results which to a large extent are not matching [3, 6, 7]. In the current work, the potentials of the composite for use in typical marine water environment are investigated. Little attention has been given to its behaviour and suitability for engineering applications in marine/ salt water environments. The behaviour of Al based composites in NaCl environment has been well reported in literature – for instance Kiourtsidis and Skolianos [8] investigated the corrosion behaviour of artificially aged T6 AA2024/SiCp composites in 3.5wt.% NaCl aqueous solution, and established that pitting primarily at the Al matrix / SiC interfaces was the mechanism of corrosion. Zhu and Hihara [9] considered the corrosion of continuous alumina-fibre reinforced Al–2 wt.% Cu–T6 metal–matrix composite in 3.15 wt.% NaCl solution, but literatures which have considered the behaviour of Al(6063) composites in NaCl can hardly be sourced. The current work aims at studying the corrosion behaviour of Al (6063) – SiC subjected to varied heat – treatment conditions with a view to establishing its suitability in marine/salt water environment which is typical of the Nigerian offshore industrial environment. The investigation will go a long way in providing useful data to ascertain the potential application of Al (6063) - SiC composites in marine environments.
Material and Method
Composite Production
Al (6063) alloy with base composition - 0.46Si, 0.23Fe, 0.02Cu, 0.03Mn, 0.51Mg, 0.02Zn,0.03Cr, 0.03Ti and the balance Al (in wt.%) was utilized as metal matrix for the development of the composite. The Al6063-SiCp composites were prepared containing 6, 12 and 15 volume percents (vol.%) SiC particles (30μm size) using charge calculations. The problem of wettability common to Al based - SiC composites were tackled by mixing the SiC with dehydrated Borax in ratio 2:1. The samples were made using two step stir casting method which involved melting the Al-6063 ingots and then cooling to a semi-solid state before introducing the SiC particulates - Borax mixture and stirring manually for 10 - 15 minutes. This was followed by heating of the mixture to 30ºC above the liquidus and then performing a second stirring using a mechanical stirrer at a revolution of 300rpm for 10 minutes before casting into rectangular block moulds. Al6063 alloy without reinforcement was also prepared for control experimentation.
Heat Treatment
Four different temper conditions were utilized in this study, the as-cast condition, and three others developed by heat-treatment, namely, solutionized and water quenched; artificially age hardened at 180ºC; and artificially age hardened at 195°C. The Solutionized and quenched temper was achieved by solutionizing the samples at 550°C for 3hours and then quenching in water. The two artificial ageing tempers were achieved by initially solutionizing at 550°C for 3hours followed by water quenching. Age hardening treatments were performed at 180°C and 195°C for 2 hours before water quenching.
Electrochemical Testing
The corrosion tests were carried out in 5wt% NaCl (pH 8.37) which was prepared using standard procedures. The specimens for the test were cut to size 20×20×5 mm, after which the sample surfaces were mechanically polished with emery papers starting from 120grit down to 640grit size. The samples were de-greased with acetone and then rinsed in distilled water before immersion in still solutions of 5wt% NaCl in deionized water exposed to atmospheric air. The solution-to-specimen surface area ratio was about 150 ml cm-2. The results of the corrosion tests were evaluated by mass loss, corrosion rate, and electrode potential measurements; and the electrochemical experiments were monitored on two day intervals for a period of 45days. Mass loss (mg/cm2) for each sample was evaluated by dividing the weight loss (measured using a four decimal digit electronic weighing balance) by its total surface area which is in accordance with ASTM G31 standard recommended practice [10]. Corrosion rate for each sample was evaluated from the weight loss measurements following standard procedures. The electrode potential measurements were also performed employing a digital multimeter and using Zn electrode as reference electrode.
Results and Discussion
Influence of SiC Volume Percent on Corrosion Behaviour
Figure 1 presents the mass loss and corrosion rate plots for the as - cast samples immersed in 5wt% NaCl solution. It is observed that mass loss was more predominant for the 6 and 12 vol% SiC reinforced composites in comparison to the monolithic alloy. The 15vol% SiC reinforced composite had the least corrosion rate of the test samples. It is observed that between the 10th to 13th days of immersion there was significant drop in mass loss which from figure 1(b) is found to be due to weight gain as the samples all had negative corrosion rates. The weight gain is as a result of the formation of passive films on the surface of the samples which lead to the temporary seizure of the corrosion attack. Corrosion attack is restored after the 13th day as is observed from figure 1(a) due to the gradual breakdown of the passive films. However, the rate of corrosion after the weight gain is considerably lower than what was observed at the earlier stages of immersion suggesting that the passive films formed on the surface of the composites were stable. Figure 1(b) shows that the corrosion rate was uniform till the end of the immersion test. The unusual high corrosion resistance of the 15vol% SiC reinforced composite could not be factored but might be due to the level of homogeneity in the as – cast structure. The more pronounced corrosion observed for the composites is attributed to the formation of localized galvanic cells at the Al matrix/SiC particulate interfaces which facilitates intensified dissolution of Al matrix in such regions [8]. However the corrosion rate is generally low for both the composites and the monolithic alloy when compared with HCl - H2SO4 environments [5].
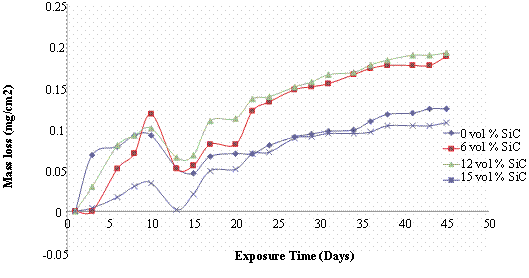
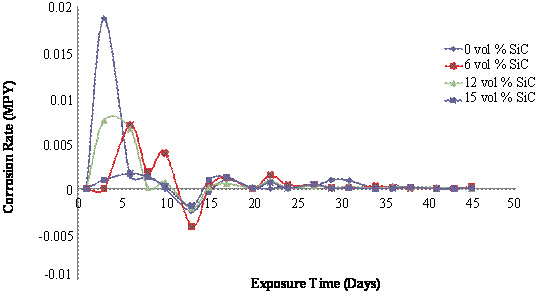
Figure 1. Mass loss and corrosion rate of as-cast Al (6063)/SiCp composites in 3.5wt% NaCl Solution
The mass loss and corrosion rate plots for the composites and monolithic alloy subjected to solution treatment and quenching operation is presented in figure 2. It is observed in this case that mass loss increased with increase in the volume percent of SiC as it is observed from figure 2(a) that the 6vol% (0.046mg/cm2) and the monolithic alloy (0.047mg/cm2) had the least mass loss, followed by the 12vol% SiC reinforced composite (0.14mg/cm2) and then the 15vol % SiC reinforced composite which had the highest mass loss value (0.25mg/cm2). However it is observed that the 6vol% SiC reinforced composite has the most intense corrosion rate at the early stages of immersion in comparison with the other composites. For all the composites it is observed that corrosion rate was more intense at the earlier stage of immersion in comparison with the monolithic alloy (Figure 2b).
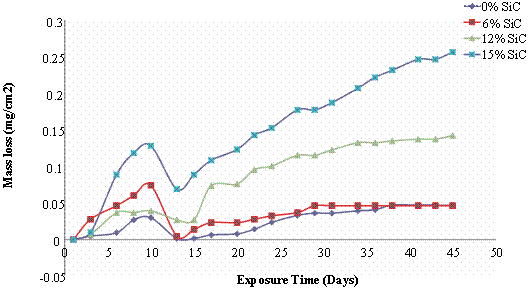
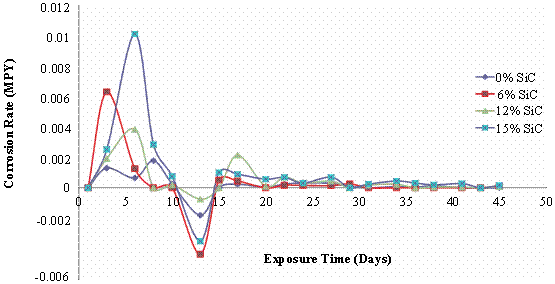
Figure 2. Mass loss and corrosion rate of solution heat-treated Al (6063)/SiCp composites in 3.5wt% NaCl Solution
Also the weight gain effect observed between the 10th to 13th days of immersion was more pronounced in the composites as reflected by the large negative corrosion rates observed. The corrosion rate thereafter was much reduced and uniform for all the samples indicating that the passive films formed on the surface of the specimens remained stable to a large extent till the completion of the immersion test.
The corrosion behaviour of the age - hardened specimens (Figures 3 and 4) to a large extent was similar to that observed for the solution treated and quenched samples.
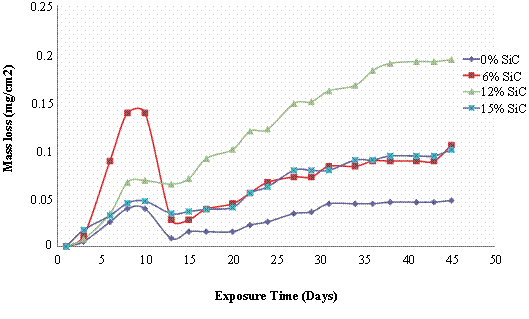
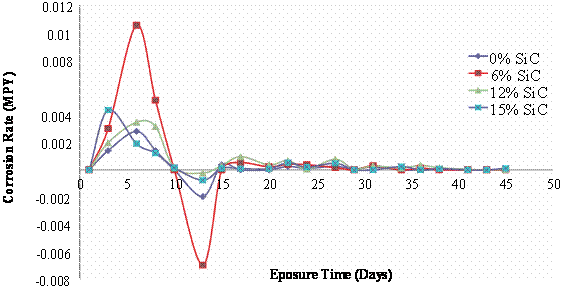
Figure 3. Mass loss and corrosion rate of 180°C age - hardened Al (6063)/SiCp composites in 3.5wt % NaCl Solution
This is with respect to the higher corrosion rates of the composites in comparison to the monolithic alloy. Again it is observed that the corrosion rate after the weight gain shows that there is a great reduction in the corrosion susceptibility indicating that the Al (6063) – SiC particulate composites can be utilized satisfactorily in marine/salt water environments.
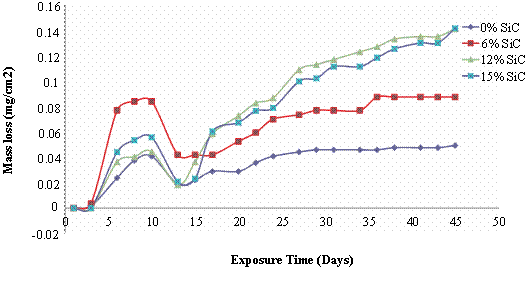
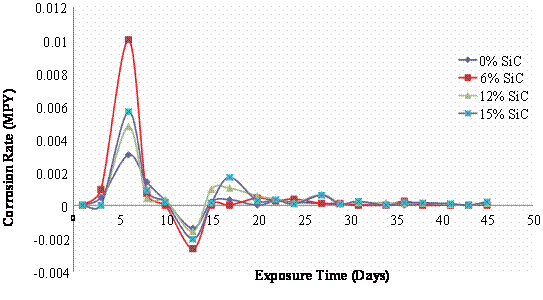
Figure 4. Mass loss and corrosion rate of 195°C age - hardened Al (6063)/SiCp composites in 3.5wt % NaCl Solution
Conclusions
The corrosion susceptibility of the Al (6063) - SiCp composites was found to be higher than that of the monolithic alloy, and for most cases the corrosion rate of the composites increased with increase in volume percent of SiC. However, it was discovered that the nature of the passive films formed on the composites was sufficiently stable to reduce significantly the corrosion rate of the composites after 13days of immersion. This trend was observed to be consistent for all heat-treatment conditions utilized.
References
3. Saraswathi Y.L., Das S., Mondal D.P., Influence of microstructure and experimental parameters on the erosion-corrosion behaviour of Al alloy composites, Materials Science and Engineering, 2006, A 425, p. 244 - 254.
4. Singla M., Dwivedi D.D., Singh L., Chawla V., Development of Aluminium Based Silicon Carbide Particulate Metal Matrix Composite, Journal of Minerals and Materials Characterization and Engineering, 2009, Vol. 8, No.6, p. 455 - 467.
6. Pinto G.M., Nayak J., Shetty A.N., Corrosion Behaviour of 6061 Al - 15 vol. Pct. SiC Composite and its Base Alloy in a Mixture of 1:1 Hydrochloric and Sulphuric Acid Medium, Int. J. Electrochem. Sci., 2009, 4, p. 1452 - 1468.