Performance Evaluation of Palm Oil as Biodiesel
Sunday Albert LAWAL1 and Ahmed BABAKANO2,*
1Department of Mechanical Engineering, Federal University of Technology, Minna, Nigeria
2Department of Mechanical Engineering, Federal University of Technology, Yola, Nigeria
E-mails: lawalbert2003@yahoo.com; ahmedba05@yahoo.com
*Corresponding author e-mail: ahmedba05@yahoo.com
Received: 8 April 2010 / Accepted: 30 March 2011 / Published: 28 June 2011
Abstract
This work involved the production of diesel from most commonly available palm fruits oil Pisifera elaeis guineensis and testing for the brake power, torque of an engine and specific fuel consumption of a conventional diesel engine utilizing the produced diesel from palm oil. The obtained results were compared with those for fossil diesel fuel. The results show that the value of brake power was 6927.21W for fossil diesel while that of biodiesel was 7135.02W. Similarly the value for brake torque for fossil diesel was 44.1Nm and that of biodiesel was 45.42Nm. The average value obtained for the specific fuel consumption in the three experiments conducted for fossil diesel and biodiesel were 69.09 and 129.21 l/kWh respectively. It was discovered that the values of brake power, torque power and specific fuel consumption for bio diesel fuel were higher than those of fossil diesel fuel.
Keywords
Biodiesel; Fuel; Engine; Renewable; Oil.
Introduction
Biodiesel is an alternative to petroleum-based diesel fuel and it is made from renewable resources such as vegetable oils, animal fats or algae. A lipid transesterification production process is used to convert the base oil to the desired ester and to remove free fatty acids [1]. After this processing, the end product, biodiesel, unlike the straight vegetable oil has combustion properties very similar to fossil diesel. Biodiesel is non-flammable and in contrast to fossil diesel, it is non-explosive, with a flash point of 150°C as compared with 64°C for fossil diesel [2].
Unlike fossil diesel, it is biodegradable and non-toxic, and it reduces toxic and other emissions when burned as a fuel. It is a clear amber-yellow liquid with a viscosity similar to fossil diesel [2].
Biodiesel can be mixed with petroleum diesel at any concentration in most modern engines, although it has the disadvantages of degrading rubber gaskets and hoses in older vehicles, vehicles prior to 1992 [2]. It is a better solvent than fossil diesel and has been known to break down deposits of residue in the fuel lines of vehicles, which usually run on petroleum. Consequently, fuel filters may become clogged with particulates if a quick transition to pure biodiesel is made, but the biodiesel cleans the engine in the process [2].
Biodiesel also eliminates sulphur emissions (SO2), because it does not include sulphur. Also, it has a higher cetane rating than fossil diesel, and therefore ignites more rapidly when injected into the engine [3]. It contains fewer aromatic like Benzo fluo ranthene and enzopyrenes.
It is one of the substitutes to replace fossil fuels as the world’s primary transport energy source, because it is a renewable fuel that can replace fossil diesel in current engines and can be transported and sold using today’s infrastructures. Diesel derived from vegetable oil have good potential as an alternative diesel fuel because it’s energy content, viscosity and phase changes are similar to those of fossil diesel [3].
It has been estimated that the annual consumption of fossil fuel is an amount that took nature, on average, about one million years to produce and since energy consumption will triple over the next 50 years, with enormous demand arising particularly in the so-called developing countries like Nigeria. This demand will include the use of carbon-free source of energy [4-7].
Bio-fuels provide us with completely emission-free energy cycle [8, 9]. The initial energy of the biomass oxygen system is captured from solar radiation in photosynthesis and when released in combustion, the biofuel energy is dissipated. The elements of the material should be available for recycling in natural, ecological, or agricultural processes. Thus, the use of industrial bio-fuels when linked carefully to natural ecological cycles may be non-polluting and sustainable [10-12]. Although there is no energy source that is completely environmentally safe, energy must be used more wisely in order to minimize the environmental hazard and optimize the efficiency with which it is produced [13-16].
One of the most interesting developments is a growing realization that vegetable oils present a practical alternative to fuels and lubricants derived from liquid fossil fuels, which is the focus of this paper.
Material and Method
Materials
The materials used for the experiment were raw palm oil, potassium hydroxide analytical pellets, methanol, vinegar (acetic acid), water, fossil diesel and potassium methoxide.
The following equipment was involved: (i) volumetric flask, (ii) beakers, (iii) thermometer, with range 0-100°C graduated in 1°C, (iv) magnetic stirrer hotplate, (v) funnels, (vi) conical flask, (vii) test tubes, (viii) separating funnels,(ix) distillation apparatus, (x) measuring cylinders, (xi) supply tank, (xii) graduated cylinder, (xiii) 3-way tap, (xiv) supply pipe, (xv) adjusting screw, (xvi) steel band, (xvii) flywheel, (xviii) stops, (xix) weights and brake lining material (hardened fibre).
Method
The transesterification reaction was carried out in a conical flask equipped with a thermometer and mounted on a magnetic stirrer hotplate. Meanwhile, 500ml of palm oil sourced from pisifera elaeis guineensis species was heated to 45°C in a container in order to improve its mixability with methanol. Agitation was provided by the magnetic stirrer which was set at constant speed. The choice of speed may vary, but for every experiment, it must be constant throughout.
Then, 2.3 grams of potassium hydroxide was dissolved in 100ml of methanol and the mixture was stirred for about 15 minutes. Potassium methoxide was then introduced into the vessel containing palm oil.
The heater was set to bring the temperature of the reaction mixture to 55°C and the system was held at this temperature while mixing proceeded for an hour.
The reacted mixture was allowed to stand for about eight hours in order for the phases to separate and settle. A separating technique namely decantation with a funnel was used to separate the waste (glycerin) from the biodiesel, which was collected in separate bottles.
Further, 10ml of acetic acid (vinegar) was added to the product sample and then washed with 100ml of water.
The mixture was left for another twenty four hours to allow the oil and water to separate. The denser soapy water was drained out through the bottom leaving the cleaned product whose volume was ascertained in a measuring cylinder [17].
Experimental Procedures for the Determination of Brake Power
A steel band with the brake lining material (hardened fibre) was placed on the flywheel and connected to a load reception unit and various sizes of dead loads (loads acting in one direction) in kilograms were used to resist the motion of the flywheel.
The engine was set on 1500 rpm, weights were placed in the load reception unit, until a point where the total load added resisted completely the motion of the flywheel.
The weight obtained at the point the flywheel stopped was noted. This weight in Newton’s was then used to calculate the brake power of the engine using Equation (1) for both fossil diesel and biodiesel fuels [18].
|
|
(1) |
where:
|
|
(2) |
and Pb = brake power in Nm/s or Watts; W or F = weight in Newton and T = torque; R = radius (distance in metre) and N = number of revolution per second.
Experimental Procedures for the Determination of Torque
The same experimental procedure for determining brake power was followed and the total loaded weight that stopped the flywheel was recorded as well as the distance from the centre of the flywheel to the centre of the total loaded weights. The recorded weights and the radius were used to calculate the torque of the engine using Equation (2) for both fossil diesel and biodiesel fuels [18].
Experimental Procedure for Determination of Specific Fuel Consumption
A simple arrangement for measuring the specific fuel consumption was put in place. When used, the volume of the bio diesel in the graduated cylinder was noted.
During the experiment, the time taken for the engine to use this quantity of fuel is recorded preferably by a stop watch. The total dead load (loads acting in one direction) to resist completely the movement of the flywheel was recorded. Also, the total volume of fuel in litres was noted as well as the value of the brake power in kilowatts.
These parameters were used in determining the specific fuel consumption for both fossil diesel and biodiesel fuels [18].
Results and Discussion
The average value for the brake power obtained for fossil diesel was 6927.21W, which is lower compared with that of biodiesel which was found to be 7135.02W.
Similarly, the average value for the engine torque for fossil diesel was found to be 44.1Nm, while that of biodiesel was found to be 45.423Nm.
The average value obtained for the specific fuel consumption of biodiesel was higher than that of fossil diesel as showed on Table 1.
Table 1. Experimental results
|
S/No |
Engine dead load W (N) |
Loading period t (s) |
Brake power Pb (W) |
Engine torque (Nm) |
Specific fuel consumption (l/kWh) |
|
Fossil diesel |
98 |
30.17 |
6927.21 |
44.1 |
69.09 |
|
Biodiesel |
100.94 |
15.67 |
7135.02 |
45.423 |
129.21 |
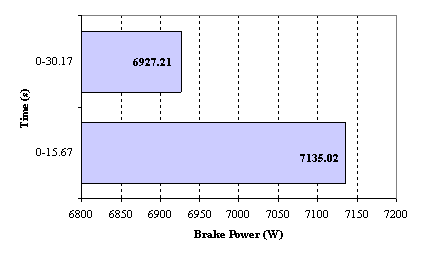
Figure 1. Brake Power developed by Fossil diesel and Biodiesel
It can be concluded that the brake power obtained while using biodiesel is biger than the one obtained while fossil fuel was used. Figure 1 show that within 15.67 second about 7135.02W was obtained using biodiesel while it took 30.17 second to obtain 6927.21W with fossil fuel.
Conclusions
The performance analysis under specific fuel consumption showed that fossil diesel had lower values than that of biodiesel indicating that biodiesel had a slightly higher viscosity compared with the fossil.
Under torque application, the biodiesel had a higher value than the fossil fuel. Also the brake power value of the biodiesel was higher than that of the fossil fuel indicating a larger brake power for the biodiesel compared with the fossil diesel.
Results obtained in this work show that bio-fuel obtained from palm oil has properties close to petrol diesel. Therefore, it can be used as a substitute for diesel oil. The palm oil has the advantage that it is a non-polluting source of energy; hence, it can help in reducing the emission of greenhouse gases and other emissions that are toxic and cacogenic.
Based on this study, it is believed that a time will be reached in the future when demand for non-polluting and efficient energy sources will be met by other sources than fossil fuel globally.
References
4. Drew H.W., General organic and biological chemistry, Philadelphia Sander College, 1995, p.40-45.
5. Wang X., Feng Z., Energy consumption with sustainable development in developing country: A case in Jiagu, China, Journal of Energy Policy, 2002, 31, p. 1671-1684.
6. Hepbasli A., Ozalp N., Development of energy efficiency and management implementation in the Turkish industrial sector, Journal of Energy Conversion Management, 2003, 44(2), p. 231-249.
9. George T.A., Shreve’s Chemical Process Industries, 5th Edition, New York, McGraw-Hill, 1984.
10. Philip M., Advanced Chemistry, London, Cambridge University Press, 1992.
11. Hall P., Planning: Millennial retrospect and prospect, Journal of Programme Planning, 2002, 57, p. 263-284.
12. Fang Y., Zeng Y., Balancing energy and environment: The effect and perspective of management instruments in China, Journal of Energy, 2007, 32(12), p. 2247-2261.
14. Zia H., Deradas V., Energy management in Lucknow City, Journal of Energy Policy, 2007, 35(10), p. 4847-4868.
15. Tsagarakis K.P., Papadogiannis C., Technical and economic evaluation of the biogas utilization for energy production at Irakilo municipality, Greece, Journal of Energy Conversion Management, 2006, 47, p. 844-857.
16. Kevin T.P., Lewis A.O., An Introduction to Global Environmental Issues, London Butler and Tanner Ltd., 1994
17. Sridharan R., Mathal I.M., Transesterification Reactions, Sri. Ind. Rcs, 1974, 33, p. 178-187.