Synthesis of 4-Thiazolidine Derivatives of 6-Nitroindazole: Pharmaceutical Importance
Pushkal SAMADHIYA*, Ritu SHARMA, Santosh K. SRIVASTAVA, and Savitri D. SRIVASTAVA
Department of Chemistry, Dr. H.S. Gour University, Sagar, M.P. India 470003
*E-mail: pushkalsamadhiya@rediffmail.com (Corresponding author)
Abstract
New series of N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(substituted phenyl)-4-oxo-5-(substitutedbenzylidene)-1,3-thiazolidine-carboxamide, compounds 5(a-j) have been synthesized. Structures of all synthesized compounds were confirmed by chemical and spectral analyses such as IR, 1H-NMR, 13C-NMR and FAB-Mass. All the final synthesized compounds 5(a-j) were screened for their antibacterial, antifungal, antitubercular and anti-inflammatory activities.
Keywords
Synthesis; 6-nitroindazole; Thiazolidinone; Antimicrobial; Antitubercular.
Introduction
Several azoles scaffold have been described in literature including indazole. Indazole derivatives are important nitrogen containing nine membered bicyclic heterocyclic compounds with applications as several biological activities and agrochemicals besides possessing important pharmacological activities such as antimicrobial [1], protein kinase inhibitors [2], antiproliferative [3], nitric oxide synthesis [4] and have also been used as anesthesia [5], antiprotozoal [6] activities. The indazole ring system is also present in many other compounds such as herbicides, dyes or sweeteners like guanidine-1H-indazole. Despite the many useful applications of indazole derivatives, indazole chemistry remains less studied compared to other heteroaromatic compounds such as indole or benzimidazole. Indazole is a ten-π electron aromatic heterocyclic system. Like the pyrazole molecule, indazole resembles both pyridine and pyrrole and its reactivity reflects this dual behaviour.
Thiazolidine ring system derives special important from the fact that it plays important role in medicinal chemistry. Substituted thiazolidine derivatives represent important key intermediates for synthesis of pharmacologically active drug thiazolidinone has wide range biological activities such as antifungal [7], antiproliferative [8], antiinflammatory [9], antimalarial [10], herbicidal [11], and antiviral properties [12].
As part of a continuing effort to develop novel heterocyclic compounds with potential therapeutic biological activities, several chemists are currently involved in the synthesis of numbers of indazole derivatives. In the present study, we have decided to synthesize a new series of compounds showed in scheme 1.
Material and Method
Melting points were taken in open capillaries and are uncorrected. Progress of reaction was monitored by silica gel-G coated TLC plates in MeOH: CHCl3 system (1:9). The spot was visualized by exposing dry plate in iodine vapours. IR spectra were recorded in KBr disc on a Schimadzu 8201 PC, FTIR spectrophotometer (nmax in cm-1) and 1H and 13C NMR spectra were measured on a Brucker DRX-300 spectrometer in CDCl3 at 300 and 75 MHz respectively using TMS as an internal standard. All chemical shifts were reported on d scales. The FAB-Mass spectra were recorded on a Jeol SX102 mass spectrometer. Elemental analyses were performed on a Carlo Erba1108 analyzer. Microwave irradiation carried out in open glass vessel. Modified microwave oven (800W) was used for the synthesis of compounds. A thermocouple used to monitor the temperature inside the vessel of the microwave. The analytical data of all the compounds were highly satisfactory. For column chromatographic purification of the products, Merck silica Gel 60 (230-400 Mesh) was used. The reagent grade chemicals were purchased from the commercial sources and further purified before use.
Method Synthesis of the Compound 1
6-Nitroindazole (0.308 mole) and 1-bromo-3-chloropropane (0.308 mole) in ethanol (100 ml) were stirred on a magnetic stirrer for about 6.30 hours at room temperature. The completion of the reaction was monitored by silica gel-G coated TLC plates. After the completion of the reaction the product was filtered and purified over a silica gel packed column chromatography using CHCl3: CH3OH (8:2 v/v) system as eluant (150 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to yield compound 1.
6-Nitroindazole (0.308 mole) and 1-bromo-3-chloropropane (0.308 mole) in ethanol (100 ml) were stirred on a magnetic stirrer for about 6.30 hours at room temperature. The completion of the reaction was monitored by silica gel-G coated TLC plates. After the completion of the reaction the product was filtered and purified over a silica gel packed column chromatography using CHCl3 : CH3OH (8 : 2 v/v) system as eluant (150 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to yield compound 1 (Figure 1).

Figure 1. Structure of compound 1
1-(3-Chloropropyl)-1H-6-nitroindazole 1. Yield: 65%; m.p. 163-165 ΊC; IR (cm-1): 768 (C-Cl), 899 (C-N), 1326 (N-CH2), 1532 (NO2), 1572 (C=C), 1448, 2842, 2889, (CH2), 3020 (CH-Ar); 1H NMR (CDCl3, 300 MHz) δ: 2.20-2.26 (m, 2H, H-9), 3.41 (t, 2H, J = 7.45 H-10), 4.26 (t, 2H, J = 7.45 Hz, H-8), 7.86-8.35 (m, 4H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 34.4 (C-9), 42.8 (C-10), 47.1 (C-8), 118.7 (C-4), 120.4 (C-7), 122.2 (C-5), 126.1 (C-3a), 136.2 (C-6), 137.0 (C-3), 135.7 (C-7a),; FAB-Mass (m/z): 239 [M+]; Anal. Calcd. for C10H10N3O2Cl: C 50.20, H 4.20, N 17.11; found C 50.17, H 4.13, N, 17.08.
Method Synthesis of the Compound 2
Compound 1 (0.208 mole) and urea (0.208 mole) in ethanol (100 ml) were stirred on a magnetic stirrer for about 4.30 hours at room temperature. The completion of the reaction was monitored by silica gel-G coated TLC plates. After the completion of the reaction the product was filtered and purified over a silica gel packed column chromatography using CHCl3:CH3OH (8:2 v/v) system as eluant (120 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to yield compound 2 (Figure 2).

Figure 2. Structure of compound 2
N-[3-(1H-6-nitroindazole-1-yl)-propyl]-urea 2. Yield: 74%; m.p. 145-147 ΊC; IR (cm-1): 752 (C-Cl), 872 (C-N), 1328 (N-CH2), 1523 (NO2), 1556 (C=C), 1648 (CO), 1435, 2839, 2910 (CH2), 3027 (CH-Ar), 3342 (NH), 3456 (NH2); 1H NMR (CDCl3, 300 MHz) δ: 2.18-2.22 (m, 2H, H-9), 3.30-3.34 (m, 2H, H-10), 4.18 (t, 2H, J = 7.45 Hz, H-8), 5.72 (s, 1H, H-1), 5.92 (s, 2, H-3), 7.34-7.96 (m, 4H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 33.1 (C-9), 42.4 (C-10), 46.7 (C-8), 117.6 (C-4), 119.3 (C-7), 121.4 (C-5), 125.8 (C-3a), 134.5 (C-6), 136.7 (C-3), 139.2 (C-7a), 161.7 (C-2); FAB-Mass (m/z): 263 [M+]; Anal. Calcd for C11H13N5O3: C 50.18, H 4.97, N 26.60; found C 50.10, H 4.92, N 26.54.
General Methods for the Synthesis of Compounds 3(a-j)
The compound 2 (0.026 mole) and benzaldehyde (0.026 mole) in ethanol (100 ml) in the presence of 2-4 drops of glacial acetic acid were first stirred on a magnetic stirrer for about 2.00 hours followed by reflux on a steam bath for about 2.30 hours. The completion of the reaction was monitored by silica gel-G coated TLC plates. The product was filtered and cooled at room temperature. The filtered product was purified over a silica gel packed column chromatography using CH3OH : CHCl3 (7 : 3 v/v) as eluant (80 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to furnish compound 3a (Figure 3).
Compounds 3 (b-j) have also been synthesized by using similar method as above.
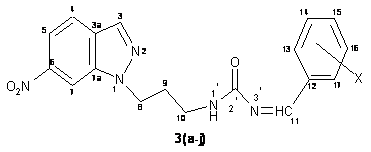
Figure 3. Structure of compound 3(a-j)
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(phenyl)-methylidene]-urea (3a). Yield: 62%; m.p. 147-148 ΊC; IR (cm-1): 742 (C-Cl), 871 (C-N), 1328 (N-CH2), 1523 (NO2), 1534 (C=C), 1555 (N=CH), 1650 (C=O), 1442, 2839, 2897 (CH2), 3027 (CH-Ar), 3356 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.22-2.27 (m, 2H, H-9), 3.24-3.27 (m, 2H, H-10), 4.14 (t, 2H, J = 7.45 Hz, H-8), 5.89 (s, 1H, H-1), 7.98 (s, 1H, H-11), 7.22-7.97 (m, 9H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 28.1 (C-9), 38.4 (C-10), 45.3 (C-8), 115.7 (C-4), 119.4 (C-7), 120.5 (C-5), 121.5 (C-3a), 121.8 (C-13 and C-17), 122.4 (C-14 and C-16), 126.5 (C-15), 128.4 (C-12), 131.3 (C-6), 132.5 (C-3), 139.2 (C-7a), 150.6 (C-11), 159.9 (C-2); FAB-Mass (m/z): 351 [M+]; Anal. Calcd. for C18H17N5O3: C 61.53, H 4.87, N 19.93; found C 61.51, H 4.80, N 19.85.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(4-chlorophenyl)-methylidene]-urea (3b). Yield: 66%; m.p. 165-167 ΊC; IR (cm-1): 746 (C-Cl), 905 (C-N), 1351 (N-CH2), 1534 (NO2), 1572 (C=C), 1580 (N=CH), 1633 (C=O), 1447, 2845, 2917 (CH2), 3014 (CH-Ar), 3344 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.23-2.28 (m, 2H, H-9), 3.40-3.45 (m, 2H, H-10), 4.26 (t, 2H, J = 7.45 Hz, H-8), 5.66 (s, 1H, H-1) 7.89 (s, 1H, H-11), 7.71-8.16 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 28.6 (C-9), 38.7 (C-10), 46.6 (C-8), 118.5 (C-4), 121.4 (C-7), 122.4 (C-5), 124.7 (C-3a), 123.9 (C-13 and C-17), 124.2 (C-14 and C-16), 127.4 (C-15), 129.5 (C-12), 132.1 (C-6), 133.5 (C-3), 141.9 (C-7a), 150.6 (C-11), 161.8 (C-2); FAB-Mass (m/z): 385 [M+]; Anal. Calcd. for C18H16N5O3Cl: C 56.03, H 4.18, N 18.15; found C 55.97, H 4.15, N 18.12.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(3-chlorophenyl)-methylidene]-urea (3c). Yield: 65%; m.p. 162-163 ΊC; IR (cm-1): 751 (C-Cl), 875 (C-N), 1337 (N-CH2), 1532 (NO2), 1543 (C=C), 1556 (N=CH), 1657 (C=O), 1431, 2848, 2890 (CH2), 3032 (CH-Ar), 3362 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.21-2.27 (m, 2H, H-9), 3.40-3.45 (m, 2H, H-10), 4.20 (t, 2H, J = 7.45 Hz, H-8), 5.62 (s, 1H, H-1), 7.90 (s, 1H, H-11), 7.75-8.20 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 31.4 (C-9), 42.5 (C-10), 48.9 (C-8), 118.9 (C-4), 120.2 (C-7), 122.7 (C-5), 123.1 (C-3a), 123.5 (C-13), 123.9 (C-17), 125.6 (C-14), 126.3 (C-16), 129.6 (C-15), 131.4 (C-12), 133.9 (C-6), 134.4 (C-3), 141.7 (C-7a), 152.5 (C-11), 162.7 (C-2); FAB-Mass (m/z): 385 [M+]; Anal. Calcd. for C18H16N5O3Cl: C 56.03, H 4.18, N 18.15; found C 55.99, H 4.17, N,18.11.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(2-chlorophenyl)-methylidene]-urea (3d). Yield: 62%; m.p. 164-165 ΊC; IR (cm-1): 746 (C-Cl), 878 (C-N), 1344 (N-CH2), 1529 (NO2), 1537 (C=C), 1566 (N=CH), 1661 (C=O), 1453, 2852, 2892 (CH2), 3034 (CH-Ar), 3360 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.31-2.35 (m, 2H, H-9), 3.40-3.47 (m, 2H, H-10), 4.21 (t, 2H, J = 7.40 Hz, H-8), 5.64 (s, 1H, H-1), 7.92 (s, 1H, H-11), 7.69-8.30 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 31.7 (C-9), 42.8 (C-10), 48.4 (C-8), 117.8 (C-4), 119.7 (C-7), 123.3 (C-5), 123.6 (C-3a), 123.9 (C-13), 124.0 (C-17), 124.6 (C-14), 124.8 (C-16), 126.6 (C-15), 130.2 (C-12), 133.8 (C-6), 134.5 (C-3), 140.1 (C-7a), 152.7 (C-11), 160.7 (C-2); Mass(FAB): 385 [M+]; Anal. Calcd. for C18H16N5O3Cl: C 56.03, H 4.18, N 18.15; found C 55.97, H 4.15, N 18.14.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(4-bromophenyl)-methylidene]-urea (3e). Yield: 64%; m.p. 159-161 ΊC; IR (cm-1): 749 (C-Cl), 881 (C-N), 1340 (N-CH2), 1526 (NO2), 1538 (C=C), 1565 (N=CH), 1656 (C=O), 1454, 2847, 2900 (CH2), 3035 (CH-Ar), 3367 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.30-2.36 (m, 2H, H-9), 3.41-3.50 (m, 2H, H-10), 4.33 (t, 2H, J = 7.50 Hz, H-8), 5.65 (s, 1H, H-1), 7.93 (s, 1H, H-11), 7.74-8.26 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 29.2 (C-9), 40.3 (C-10), 47.5 (C-8), 116.7 (C-4), 121.4 (C-7), 121.8 (C-57), 122.5 (C-13 and C-17), 122.9 (C-3a), 123.7 (C-14 and C-16), 127.3 (C-15), 129.1 (C-12), 131.6 (C-6), 132.9 (C-3), 142.4 (C-7a), 153.7 (C-11), 160.0 (C-2); FAB-Mass (m/z): 430 [M+]; Anal. Calcd. for C18H16N5O3Br: C 50.24, H 3.74, N 16.27; found C 50.21, H 3.71, N 16.22.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(3-bromophenyl)-methylidene]-urea (3f). Yield: 68%; m.p. 157-158 ΊC; IR (cm-1): 755 (C-Cl), 874 (C-N), 1334 (N-CH2), 1532 (NO2), 1541 (C=C), 1570 (N=CH), 1662 (C=O), 1445, 2846, 2891 (CH2), 3042 (CH-Ar), 3365 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.21-2.29 (m, 2H, H-9), 3.42-3.50 (m, 2H, H-10), 4.28 (t, 2H, J = 7.45 Hz, H-8), 5.59 (s, 1H, H-1), 7.94 (s, 1H, H-11), 7.79-8.29 (m, 8H, Ar-H);13C NMR (CDCl3, 75 MHz) δ: 29.5 (C-9), 32.7 (C-10), 46.3 (C-8), 117.9 (C-4), 120.4 (C-7), 121.5 (C-5), 123.1 (C-3a), 124.3 (C-13), 124.8 (C-17), 125.4 (C-14), 126.3 (C-16), 129.6 (C-15), 130.7 (C-12), 131.8 (C-6), 132.9 (C-3), 141.2 (C-7a), 154.6 (C-11), 162.3 (C-2); FAB-Mass (m/z): 430 [M+]; Anal. Calcd. for C18H16N5O3Br: C 50.24, H 3.74, N 16.27; found C 50.18, H 3.72, N 16.23.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(2-bromophenyl)-methylidene]-urea (3g). Yield: 69%; m.p. 159-160 ΊC; IR (cm-1): 752 (C-Cl), 876 (C-N), 1335 (N-CH2), 1531 (NO2), 1544 (C=C), 1567 (N=CH), 1654 (C=O), 1446, 2853, 2893 (CH2), 3037 (CH-Ar), 3370 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.21-2.28 (m, 2H, H-9), 3.50-3.55 (m, 2H, H-10), 4.29 (t, 2H, J = 7.50 Hz, H-8), 5.60 (s, 1H, H-1), 7.97 (s, 1H, H-11), 7.81-8.25 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 29.9 (C-9), 32.1 (C-10), 45.8 (C-8), 117.3 (C-4), 120.4 (C-7), 122.6 (C-5), 124.1 (C-3a), 124.5 (C-13), 124.9 (C-17), 125.6 (C-14), 126.1 (C-16), 129.9 (C-15), 131.4 (C-12), 134.3 (C-6), 135.4 (C-3), 142.6 (C-7a), 153.7 (C-11), 159.5 (C-2); FAB-Mass (m/z): 430 [M+]; Anal. Calcd. for C18H16N5O3Br: C 50.24, H 3.74, N 16.27; found C 50.18, H 3.70, N 16.21.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(4-nitrophenyl)-methylidene]-urea (3h). Yield: 62%; m.p. 162-163 ΊC; IR (cm-1): 750 (C-Cl), 877 (C-N), 1339 (N-CH2), 1527 (NO2), 1536 (C=C), 1560 (N=CH), 1663 (C=O), 1455, 2845, 2898 (CH2), 3038 (CH-Ar), 3357 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.24-2.30 (m, 2H, H-9), 3.46-3.53 (m, 2H, H-10), 4.36 (t, 2H, J = 7.40 Hz, H-8), 5.61 (s, 1H, H-1), 7.98 (s, 1H, H-11), 7.84-8.22 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 30.3 (C-9), 44.4 (C-10), 46.0 (C-8), 115.5 (C-4), 122.9 (C-7), 123.4 (C-5), 123.8 (C-3a), 124.3 (C-13 and C-17), 124.6 (C-14 and C-16), 128.4 (C-15), 130.4 (C-12), 132.7 (C-6), 133.7 (C-3), 141.5 (C-7a), 154.6 (C-11), 161.9 (C-2); FAB-Mass (m/z): 396 [M+]; Anal. Calcd. for C18H16N6O5: C 54.54, H 4.06, N 21.20; found C 54.51, H 4.02, N 21.17.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(3-nitrophenyl)-methylidene]-urea (3i). Yield: 64%; m.p. 166-167 ΊC; IR (cm-1): 748 (C-Cl), 882 (C-N), 1336 (N-CH2), 1535 (NO2), 1540 (C=C), 1563 (N=CH), 1658 (C=O), 1456, 2850, 2896 (CH2), 3040 (CH-Ar), 3358 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.30-2.36 (m, 2H, H-9), 3.50-3.54 (m, 2H, H-10), 4.37 (t, 2H, J = 7.40 Hz, H-8), 5.63 (s, 1H, H-1), 7.95 (s, 1H, H-11), 7.73-8.27 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 30.6 (C-9), 41.5 (C-10), 47.8 (C-8), 116.4 (C-4), 120.9 (C-7), 121.9 (C-5), 122.5 (C-3a), 123.4 (C-13), 123.7 (C-17), 124.8 (C-14), 125.7 (C-16), 128.1 (C-15), 131.5 (C-12), 133.9 (C-6), 135.6 (C-3), 140.6 (C-7a), 154.3 (C-11), 161.9 (C-2); FAB-Mass (m/z): 396 [M+]; Anal. Calcd. for C18H16N6O5: C 54.54, H 4.06, N 21.20; found C 54.49, H 4.03, N 21.16.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-N-[(2-nitrophenyl)-methylidene]-urea (3j). Yield: 66%; m.p. 160-161 ΊC; IR (cm-1): 753 (C-Cl), 879 (C-N), 1338 (N-CH2), 1526 (NO2), 1542 (C=C), 1557 (N=CH), 1660 (C=O), 1447, 2851, 2901 (CH2), 3036 (CH-Ar), 3369 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.25-2.31 (m, 2H, H-9), 3.42-3.49 (m, 2H, H-10), 4.25 (t, 2H, J = 7.50 Hz, H-8), 5.58 (s, 1H, H-1), 7.93 (s, 1H, H-11), 7.76-8.28 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 30.8 (C-9), 40.9 (C-10), 47.2 (C-8), 116.6 (C-4), 120.6 (C-7), 121.9 (C-5), 122.1 (C-13), 122.5 (C-3a), 122.9 (C-17), 123.5 (C-14), 123.9 (C-16), 127.2 (C-15), 128.6 (C-12), 131.6 (C-6), 132.9 (C-3), 139.7 (C-7a), 154.1 (C-11), 162.2 (C-2); FAB-Mass (m/z): 396 [M+]; Anal. Calcd. for C18H16N6O5: C 54.54, H 4.06, N 21.20; found C 54.47, H 4.04, N 21.12.
General Methods for the Synthesis of Compounds 4(a-j)
The compound 3a (0.011 mole) and thioglycolic acid (0.011 mole) in ethanol (50 ml) in the presence of ZnCl2 were allowed to react at room temperature. The reaction mixture was first stirred on a magnetic stirrer for about 2.30 hours followed by reflux on a steam bath for about 5.00 hours. The completion of the reaction was monitored by silica gel-G coated TLC plates. The product was filtered and cooled at room temperature. The filtered product was purified over a silica gel packed column chromatography using CH3OH : CHCl3 (7 : 3 v/v) as eluant (70 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to furnish compound 4a (Figure 4).
Compounds 4(b-j) have also been synthesized by using similar method as above.
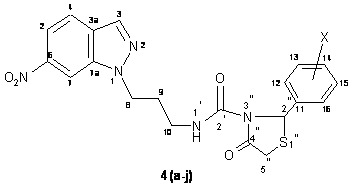
Figure 4. Structure of compound 4(a-j)
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(phenyl)-4-oxo-1,3-thiazolidine-carboxamide (4a). Yield: 65%; m.p. 160-161 ΊC; IR (cm-1): 663 (C-S-C), 880 (C-N), 1339 (N-CH2), 1531 (NO2), 1545 (C=C), 1661 (C=O), 1737 (CO cyclic), 1452, 2848, 2896 (CH2), 2958 (S-CH2), 3033, (CH-Ar), 3362 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.26-2.32 (m, 2H, H-9), 3.28-3.33 (m, 2H, H-10), 3.40 (s, 2H, H-5), 4.21 (t, 2H, J = 7.45 Hz, H-8), 5.21 (s, 1H, H-2), 5.62 (s, 1H, H-1), 7.12-8.01 (m, 9H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 30.2 (C-9), 33.5 (C-5), 39.4 (C-10), 47.5 (C-8), 56.1 (C-2), 117.3 (C-4), 122.5 (C-7), 123.3 (C-5), 128.6 (C-3a), 127.3 (C-12 and C-16), 129.7 (C-14), 130.1 (C-12 and C-15), 133.6 (C-6), 134.5 (C-3), 136.2 (C-11), 142.1 (C-7a), 161.6 (C-2), 169.8 (C-4); FAB-Mass (m/z): 425 [M+]; Anal. Calcd. for C20H19N5O4S: C, 56.46, H, 4.50, N, 16.46 %; found C, 56.41, H, 4.43, N, 16.39 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-chlorophenyl)-4-oxo-1,3-thiazolidine-carboxamide (4b). Yield: 67; m.p. 189-190 ΊC; IR (cm-1): 667 (C-S-C), 882 (C-N), 1345 (N-CH2), 1532 (NO2), 1550 (C=C), 1662 (C=O), 1742 (CO cyclic), 1458, 2851, 2899 (CH2), 2962 (S-CH2), 3040 (CH-Ar), 3366 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.20-2.24 (m, 2H, H-9), 3.32 (s, 2H, H-5), 3.35-3.39 (m, 2H, H-10), 4.28 (t, 2H, J = 7.45 Hz, H-8), 5.23 (s, 1H, H-2), 5.72 (s, 1H, H-1), 7.23-7.74 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 33.8 (C-9), 39.2 (C-5), 46.6 (C-10), 50.9 (C-8), 62.4 (C-2), 120.4 (C-4), 124.8 (C-7), 127.6 (C-5), 131.5 (C-3a), 132.3 (C-12 and C-16), 133.8 (C-14), 134.4 (C-13 and C-15), 136.7 (C-6), 139.7 (C-11), 140.9 (C-3), 144.9 (C-7a), 163.0 (C-2), 171.7 (C-4); FAB-Mass (m/z): 460 [M+]; Anal. Calcd. for C20H18N5O4SCl: C, 52.23, H, 3.94, N, 15.22 %; found C, 52.19, H, 3.89, N, 15.18 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-chlorophenyl)-4-oxo-1,3-thiazolidine-carboxamide (4c). Yield: 68%; m.p. 185-186 ΊC; IR (cm-1): 671 (C-S-C), 884 (C-N), 1341 (N-CH2), 1536 (NO2), 1552 (C=C), 1665 (C=O), 1745 (CO cyclic), 1462, 2853, 2903 (CH2), 2958 (S-CH2), 3035 (CH-Ar), 3365 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.24-2.29 (m, 2H, H-9), 3.40 (s, 2H, H-5), 3.43-3.48 (m, 2H, H-10), 4.22 (t, 2H, J = 7.50 Hz, H-8), 5.24 (s, 1H, H-2), 5.73 (s, 1H, H-1), 7.30-7.80 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 31.1 (C-9), 34.5 (C-5), 43.8 (C-10), 50.9 (C-8), 60.1 (C-2), 120.3 (C-4), 125.4 (C-7), 126.6 (C-5), 130.7 (C-3a), 131.2 (C-12), 131.9 (C-16), 133.4 (C-14), 134.6 (C-13), 135.2 (C-15), 137.3 (C-6), 137.9 (C-3),139.4 (C-11), 142.7 (C-7a),161.9 (C-2), 172.5 (C-4); FAB-Mass (m/z): 460 [M+]; Anal. Calcd. for C20H18N5O4SCl: C, 52.23, H, 3.94, N, 15.22 %; found C, 52.17, H, 3.91, N, 15.19 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-chloro phenyl)-4-oxo-1,3-thiazolidine-carboxamide (4d). Yield: 64%; m.p. 180-181 ΊC; IR (cm-1): 672 (C-S-C), 885 (C-N), 1342 (N-CH2), 1540 (NO2), 1547 (C=C), 1670 (C=O), 1749 (CO cyclic), 1453, 2849, 2904 (CH2), 2963 (S-CH2), 3036 (CH-Ar), 3372 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.23-2.28 (m, 2H, H-9), 3.38 (s, 2H, H-5), 3.41-3.46 (m, 2H, H-10), 4.29 (t, 2H, J = 7.45 Hz, H-8), 5.21 (s, 1H, H-2), 5.74 (s, 1H, H-1), 7.36-7.86 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 31.6 (C-9), 34.5 (C-5), 42.7 (C-10), 50.4 (C-8), 60.9 (C-2), 119.9 (C-4), 124.6 (C-7), 125.6 (C-5), 130.2 (C-3a), 130.7 (C-12), 131.1 (C-16), 132.6 (C-14), 133.8 (C-13), 134.6 (C-15), 135.5 (C-6), 136.4 (C-3), 138.1 (C-11), 139.9 (C-7a), 164.6 (C-2), 172.7 (C-4); FAB-Mass (m/z): 460 [M+]; Anal. Calcd. for C20H18N5O4SCl: C, 52.23, H, 3.94, N, 15.22 %; found C, 52.22, H, 3.90, N, 15.20%.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-bromo phenyl)-4-oxo-1,3-thiazolidine-carboxamide (4e). Yield: 65%; m.p. 174-175 ΊC; IR (cm-1): 670 (C-S-C), 886 (C-N), 1340 (N-CH2), 1542 (NO2), 1555 (C=C), 1671 (C=O), 1750 (CO cyclic), 1454, 2855, 2905 (CH2), 2964 (S-CH2), 3038 (CH-Ar), 3371 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.27-2.34 (m, 2H, H-9), 3.40 (s, 2H, H-5), 3.42-3.47 (m, 2H, H-10), 4.31 (t, 2H, J = 7.45 Hz, H-8), 5.22 (s, 1H, H-2), 5.79 (s, 1H, H-1), 7.38-7.83 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 30.7 (C-9), 35.4 (C-5), 42.3 (C-10), 49.1 (C-8), 59.6 (C-2), 117.5 (C-4), 122.8 (C-7), 123.5 (C-5), 129.7 (C-3a), 130.5 (C-12 and C-16), 132.3 (C-14), 133.7 (C-13 and C-15), 135.2 (C-6), 136.8 (C-3), 138.5 (C-11), 141.3 (C-7a),162.6 (C-2), 175.7 (C-4); FAB-Mass (m/z): 504 [M+]; Anal. Calcd. for C20H18N5O4SBr: C, 47.62, H, 3.52, N, 13.88 %; found C, 47.57, H, 3.45, N, 13.83 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-bromo phenyl)-4-oxo-1,3-thiazolidine-carboxamide (4f). Yield: 67%; m.p. 178-179 ΊC; IR (cm-1): 665 (C-S-C), 888 (C-N), 1346 (N-CH2), 1534 (NO2), 1556 (C=C), 1664 (C=O), 1738 (CO cyclic), 1455, 2860, 2906 (CH2), 2960 (S-CH2), 3034 (CH-Ar), 3365 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.20-2.25 (m, 2H, H-9), 3.28 (s, 2H, H-5), 3.31-3.36 (m, 2H, H-10), 4.30 (t, 2H, J = 7.50 Hz, H-8), 5.20 (s, 1H, H-2), 5.80 (s, 1H, H-1), 7.25-7.79 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 32.8 (C-9), 35.7 (C-5), 40.4 (C-10), 47.7 (C-8), 58.5 (C-2), 117.8 (C-4), 123.4 (C-7), 124.3 (C-5), 129.8 (C-3a), 128.6 (C-12), 129.3 (C-16), 130.4 (C-14), 131.5 (C-13), 133.2 (C-6), 134.9 (C-3), 133.4 (C-15), 136.5 (C-11), 142.4 (C-7a), 165.3 (C-2), 173.6 (C-4); FAB-Mass (m/z): 504 [M+]; Anal. Calcd. for C20H18N5O4SBr: C, 47.62, H, 3.52, N, 13.88 %; found C, 47.61, H, 3.45 N, 13.81 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-bromo phenyl)-4-oxo-1,3-thiazolidine-carboxamide (4g). Yield: 68%; m.p. 172-173 ΊC; IR (cm-1): 668 (C-S-C), 890 (C-N), 1348 (N-CH2), 1537 (NO2), 1553 (C=C), 1663 (C=O), 1740 (CO cyclic), 1461, 2855, 2910 (CH2), 2963 (S-CH2), 3041 (CH-Ar), 3366 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.22-2.26 (m, 2H, H-9), 3.35 (s, 2H, H-5), 3.42-3.46 (m, 2H, H-10), 4.25 (t, 2H, J = 7.40 Hz, H-8), 5.19 (s, 1H, H-2), 5.77 (s, 1H, H-1), 7.26-7.77 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 34.4 (C-9), 36.8 (C-5), 39.9 (C-10), 48.4 (C-8), 59.5 (C-2), 118.6 (C-4), 125.5 (C-7), 126.6 (C-5), 129.7 (C-12), 130.2 (C-16), 130.5 (C-3a), 131.6 (C-14), 132.7 (C-13), 133.1 (C-15), 134.8 (C-6), 135.7 (C-3), 137.5 (C-11), 140.7 (C-7a),165.8 (C-2), 173.3 (C-4); FAB-Mass (m/z): 504 [M+]; Anal. Calcd. for C20H18N5O4SBr: C, 47.62, H, 3.52, N, 13.88 %; found C, 47.59, H, 3.48, N, 13.79 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-nitrophenyl)-4-oxo-1,3-thiazolidine-carboxamide (4h). Yield: 66%; m.p. 177-179 ΊC; IR (cm-1): 673 (C-S-C), 893 (C-N), 1349 (N-CH2), 1538 (NO2), 1549 (C=C), 1666 (C=O), 1746 (CO cyclic), 1459, 2854, 2897 (CH2), 2955 (S-CH2), 3036 (CH-Ar), 3368 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.20-2.25 (m, 2H, H-9), 3.39 (s, 2H, H-5), 3.43-3.49 (m, 2H, H-10), 4.21 (t, 2H, J = 7.45 Hz, H-8), 5.36 (s, 1H, H-2), 5.70 (s, 1H, H-1), 7.26-7.84 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 32.2 (C-9), 36.9 (C-5), 40.3 (C-10), 51.8 (C-8), 57.2 (C-2), 118.6 (C-4), 123.4 (C-5), 124.7 (C-7), 127.6 (C-12 and C-16), 128.8 (C-3a), 129.9 (C-14), 130.4 (C-13 and C-15), 135.7 (C-11), 132.6 (C-6), 133.7 (C-3), 143.1 (C-7a), 162.4 (C-2), 174.3 (C-4); FAB-Mass (m/z): 470 [M+]; Anal. Calcd. for C20H18N6O6S: C, 51.06, H, 3.85, N, 17.86 %; found C, 51.00, H, 3.78, N, 17.79 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-nitrophenyl)-4-oxo-1,3-thiazolidine-carboxamide (4i). Yield: 64%; m.p. 180-181 ΊC; IR (cm-1): 666 (C-S-C), 887 (C-N), 1350 (N-CH2), 1539 (NO2), 1546 (C=C), 1669 (C=O), 1747 (CO cyclic), 1456, 2850, 2905 (CH2), 2957 (S-CH2), 3037 (CH-Ar), 3369 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.19-2.24 (m, 2H, H-9), 3.28-3.33 (m, 2H, H-10), 3.37 (s, 2H, H-5), 4.20 (t, 2H, J = 7.40 Hz, H-8), 5.41 (s, 1H, H-2), 5.69 (s, 1H, H-1), 7.29-7.81 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 33.4 (C-9), 37.4 (C-5), 41.6 (C-10), 49.7 (C-8), 58.1 (C-2), 119.6 (C-4), 124.8 (C-7), 125.6 (C-5), 128.4 (C-12), 129.5 (C-3a), 129.7 (C-16), 130.7 (C-14), 131.6 (C-13), 133.5 (C-6), 133.7 (C-15), 134.2 (C-3), 136.8 (C-11), 142.7 (C-7a), 163.4 (C-2), 174.5 (C-4); FAB-Mass (m/z): 470 [M+]; Anal. Calcd. for C20H18N6O6S : C, 51.06, H, 3.85, N, 17.86 %; found C, 51.04, H, 3.80, N, 17.81%.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-nitrophenyl)-4-oxo-1,3-thiazolidine-carboxamide (4j). Yield: 68%; m.p. 178-179 ΊC; IR (cm-1): 675 (C-S-C), 889 (C-N), 1347 (N-CH2), 1541 (NO2), 1548 (C=C), 1668 (C=O), 1748 (CO cyclic), 1460, 2852, 2902 (CH2), 2965 (S-CH2), 3040 (CH-Ar), 3370 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.24-2.29 (m, 2H, H-9), 3.29 (s, 2H, H-5), 3.34-3.39 (m, 2H, H-10), 4.26 (t, 2H, J = 7.50 Hz, H-8), 5.22 (s, 1H, H-2), 5.72 (s, 1H, H-1), 7.34-7.92 (m, 8H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 34.7 (C-9), 37.1 (C-5), 41.5 (C-10), 48.6 (C-8), 60.4 (C-2), 119.7 (C-4), 126.2 (C-7), 127.3 (C-5), 129.6 (C-12), 130.4 (C-3a), 130.7 (C-16), 131.3 (C-14), 132.8 (C-13), 134.1 (C-15), 135.3 (C-3), 136.9 (C-6), 137.3 (C-11), 142.8 (C-7a), 163.1 (C-2), 175.7 (C-4); FAB-Mass (m/z): 470 [M+]; Anal. Calcd. for C20H18N6O6S: C, 51.06, H, 3.85, N, 17.86 %; found C, 51.01, H, 3.82, N, 17.85 %.
General Methods for the Synthesis of Compounds 5(a-j)
The compound 4a (0.007 mole) and benzaldehyde (0.007 mole) in ethanol (50 ml) in the presence of CH3CH2ONa were allowed to react at room temperature. The reaction mixture was first stirred on a magnetic stirrer for about 2.00 hours followed by reflux on a steam bath for about 4.30 hours. The completion of the reaction was monitored by silica gel-G coated TLC plates. The product was filtered and cooled at room temperature. The filtered product was purified over a silica gel packed column chromatography using CH3OH : CHCl3 (7 : 3 v/v) as eluant as eluant (50 ml). The purified product was dried under vacuo and recrystallized from acetone at room temperature to furnish compound 5a (Figure 5).
Compounds 5 (b-j) have also been synthesized by using similar method as above.
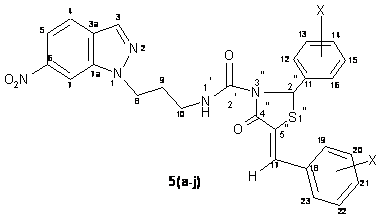
Figure 5. Structure of compound 5(a-j)
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(phenyl)-4-oxo-5-(benzylidene)-1,3-thiazolidine-carboxamide (5a). Yield: 62%; m.p. 162-163 ΊC; IR (cm-1): 670 (C-S-C), 885 (C-N), 1343 (N-CH2), 1536 (NO2), 1666 (C=O), 1741 (CO cyclic), 1457, 2854, 2902 (CH2), 2941 (C=CH), 3038 (CH-Ar), 3366 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.12-2.16 (m, 2H, H-9), 3.43-3.49 (m, 2H, H-10), 4.16 (t, 2H, J = 7.40 Hz, H-8), 5.10 (s, 1H, H-2), 5.69 (s, 1H, H-1), 6.52 (s, 1H, H-17), 7.77-8.31 (m, 14H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 32.8 (C-9), 40.1 (C-10), 54.3 (C-8), 60.5 (C-2), 117.6 (C-4), 123.2 (C-7), 125.4 (C-5), 127.3 (C-19 and C-23), 128.1 (C-12 and C-16), 129.2 (C-3a), 129.7 (C-20 and C-22), 130.5 (C-14), 131.4 (C-13 and C-15), 132.1 (C-18), 134.8 (C-6), 135.7 (C-3), 137.6 (C-11), 138.2 (C-17), 140.7 (C-5), 142.3 (C-7a), 162.0 (C-2), 170.7 (C-4); FAB-Mass (m/z): 513 [M+]; Anal. Calcd. for C27H23N5O4S: C, 63.14, H, 4.51, N, 13.63 %; found C, 63.12, H, 4.49, N, 13.57 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-chlorophenyl)-4-oxo-5-(4-chloro benzylidene)-1,3-thiazolidine-carboxamide (5b). Yield: 64%; m.p. 179-180 ΊC; IR (cm-1): 672 (C-S-C), 744 (C-Cl), 887 (C-N), 1345 (N-CH2), 1544 (NO2), 1566 (C=CH), 1672 (C=O), 1742 (CO cyclic), 1462, 2861, 2906 (CH2), 2947 (C=CH), 3040 (CH-Ar), 3370 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.15-2.20 (m, 2H, H-9), 3.45-3.51 (m, 2H, H-10), 4.20 (t, 2H, J = 7.40 Hz, H-8), 5.16 (s, 1H, H-2), 5.75 (s, 1H, H-1), 6.57 (s, 1H, H-17), 7.79-8.37 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 32 (C-9), 40.6 (C-10), 54.9 (C-8), 60.7 (C-2), 118.2 (C-4), 123.5 (C-7), 126.5 (C-5), 127.7 (C-19 and C-23), 128.4 (C-12 and C-16), 129.4 (C-3a), 129.8 (C-20 and C-22), 131.3 (C-14), 131.9 (C-13 and C-15), 132.6 (C-18), 136.4 (C-3), 134.4 (C-6), 137.6 (C-11), 141.2 (C-17), 142.3 (C-5), 142.9 (C-7a), 162.5 (C-2), 171.1 (C-4); FAB-Mass (m/z): 582 [M+]; Anal. Calcd. for C27H21N5O4SCl2: C, 55.67, H, 3.63, N, 12.02 %; found C, 55.57, H, 3.55, N, 11.95 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-chlorophenyl)-4-oxo-5-(3-chloro benzylidene)-1,3-thiazolidine-carboxamide (5c). Yield: 62%; m.p. 175-176 ΊC; IR (cm-1): 670 (C-S-C), 749 (C-Cl), 888 (C-N), 1346 (N-CH2), 1530 (NO2), 1578 (C=CH) 1647 (C=O), 1743 (CO cyclic), 1450, 2839, 2923 (CH2), 2946 (C=CH), 3047 (CH-Ar), 3362 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.19-2.24 (m, 2H, H-9), 3.46-3.50 (m, 2H, H-10), 4.15 (t, 2H, J = 7.50 Hz, H-8), 5.22 (s, 1H, H-2), 5.74 (s, 1H, H-1), 6.65 (s, 1H, H-17), 7.85-8.39 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 35.8 (C-9), 44.1 (C-10), 49.3 (C-8), 60.9 (C-2), 122.6 (C-4), 124.2 (C-7), 128.4 (C-5), 128.7 (C-19), 128.9 (C-23), 129.1 (C-12), 129.8 (C-16), 130.2 (C-3a), 130.7 (C-20), 131.2 (C-22), 131.8 (C-14), 132.2 (C-13), 132.7 (C-15), 133.1 (C-18), 135.3 (C-6), 136.6 (C-3), 138.6 (C-11), 140.6 (C-17), 141.3 (C-7a), 143.7 (C-5), 166.0 (C-2), 173.4 (C-4); Mass (FAB) 582 [M+]; Anal. Calcd. for C27H21N5O4SCl2: C, 55.67, H, 3.63, N, 12.02 %; found C, 55.59, H, 3.60, N, 11.98 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-chloro phenyl)-4-oxo-5-(2-chloro benzylidene)-1,3-thiazolidine-carboxamide (5d). Yield: 68%; m.p. 172-173 ΊC; IR (cm-1): 676 (C-S-C), 741 (C-Cl), 890 (C-N), 1347 (N-CH2), 1541 (NO2), 1568 (C=CH), 1667 (C=O), 1744 (CO cyclic), 1464, 2856, 2903 (CH2), 2949 (C=CH), 3042 (CH-Ar), 3372 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.22-2.27 (m, 2H, H-9), 3.54-3.59 (m, 2H, H-10), 4.22 (t, 2H, J = 7.50 Hz, H-8), 5.18 (s, 1H, H-2), 5.77 (s, 1H, H-1), 6.67 (s, 1H, H-17), 7.83-8.40 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 36.5 (C-9), 43.4 (C-10), 50.0 (C-8), 57.3 (C-2), 119.6 (C-4), 125.5 (C-7), 126.4 (C-5), 128.3 (C-19), 128.7 (C-23), 129.4 (C-12), 129.7 (C-16), 130.2 (C-3a), 130.9 (C-20), 131.1 (C-22), 131.7 (C-14), 132.4 (C-13), 132.8 (C-15), 133.6 (C-18), 135.8 (C-6), 137.7 (C-3), 139.6 (C-11), 141.5 (C-17), 141.7 (C-7a), 143.8 (C-5), 163.7 (C-2), 171.8 (C-4); FAB-Mass (m/z): 582 [M+]; Anal. Calcd. for C27H21N5O4SCl2: C, 55.67, H, 3.63, N, 12.02 %; found C, 55.64, H, 3.57, N, 12.00 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-bromo phenyl)-4-oxo-5-(4-bromo benzylidene)-1,3-thiazolidine-carboxamide (5e). Yield: 64%; m.p. 169-171 ΊC; IR (cm-1): 679 (C-S-C), 891 (C-N), 1352 (N-CH2), 1542 (NO2), 1572 (C=CH), 1670 (C=O), 1746 (CO cyclic), 1465, 2859, 2904 (CH2), 2945 (C=CH), 3044 (CH-Ar), 3375 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.23-2.28 (m, 2H, H-9), 3.49-3.55 (m, 2H, H-10), 4.27 (t, 2H, J = 7.40 Hz, H-8), 5.15 (s, 1H, H-2), 5.78 (s, 1H, H-1), 6.68 (s, 1H, H-17), 7.81-8.32 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 33.7 (C-9), 41.6 (C-10), 51.3 (C-8), 61.7 (C-2), 121.6 (C-4), 123.6 (C-7), 125.5 (C-5), 129.3 (C-19 and C-23), 130.3 (C-12 and C-16), 130.9 (C-3a), 131.7 (C-20 and C-22), 132.5 (C-14), 133.6 (C-13 and C-15), 134.5 (C-18), 136.6 (C-6), 138.7 (C-3), 139.7 (C-17), 140.4 (C-11), 141.6 (C-7a), 143.5 (C-5), 163.8 (C-2), 171.5 (C-4); Mass (FAB) 671 [M+]; Anal. Calcd. for C27H21N5O4SBr2: C, 48.30, H, 3.15, N, 10.43 %; found C, 48.21, H, 3.14, N, 10.39 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-bromophenyl)-4-oxo-5-(3-bromo benzylidene)-1,3-thiazolidine-carboxamide (5f). Yield: 61%; m.p. 173-174 ΊC; IR (cm-1): 680 (C-S-C), 892 (C-N), 1348 (N-CH2), 1538 (NO2), 1573 (C=CH), 1671 (C=O), 1748 (CO cyclic), 1466, 2860, 2905 (CH2), 2944 (C=CH), 3046 (CH-Ar), 3367 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.22-2.29 (m, 2H, H-9), 3.47-3.52 (m, 2H, H-10), 4.24 (t, 2H, J = 7.45 Hz, H-8), 5.23 (s, 1H, H-2), 5.78 (s, 1H, H-1), 6.58 (s, 1H, H-17), 7.89-8.36 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 35.6 (C-9), 41.4 (C-10), 48.8 (C-8), 61.5 (C-2), 119.4 (C-4), 125.5 (C-7), 127.4 (C-5), 129.8 (C-19), 130.0 (C-23), 130.3 (C-12), 130.7 (C-16), 131.4 (C-3a), 131.7 (C-20), 132.0 (C-22), 132.5 (C-14), 133.4 (C-13), 133.8 (C-15), 134.7 (C-18), 136.6 (C-6), 137.2 (C-3), 138.3 (C-11), 140.6 (C-17), 141.6 (C-7a), 142.7 (C-5), 164.6 (C-2), 174.7 (C-4); FAB-Mass (m/z): 671 [M+]; Anal. Calcd. for C27H21N5O4SBr2: C, 48.30, H, 3.15, N, 10.43 %; found C, 48.27, H, 3.10, N, 10.40 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-bromophenyl)-4-oxo-5-(2-bromo benzylidene)-1,3-thiazolidine-carboxamide (5g). Yield: 63%; m.p. 165-166 ΊC; IR (cm-1): 674 (C-S-C), 885 (C-N), 1343 (N-CH2), 1536 (NO2), 1565 (C=CH) 1666 (C=O), 1741 (CO cyclic), 1457, 2854, 2902 (CH2), 2941 (C=CH), 3038 (CH-Ar), 3366 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.21-2.28 (m, 2H, H-9), 3.50-3.55 (m, 2H, H-10), 4.25 (t, 2H, J = 7.45 Hz, H-8), 5.27 (s, 1H, H-2), 5.81 (s, 1H, H-1), 6.62 (s, 1H, H-17), 7.84-8.42 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 34.4 (C-9), 42.4 (C-10), 49.9 (C-8), 59.4 (C-2), 121.2 (C-4), 124.8 (C-7), 126.3 (C-5), 130.6 (C-19), 130.8 (C-23), 131.1 (C-12), 131.7 (C-16), 132.5 (C-3a), 132.8 (C-20), 133.4 (C-22), 133.8 (C-14), 134.4 (C-13), 134.8 (C-15), 135.5 (C-18), 137.7 (C-6), 138.9 (C-3), 139.4 (C-11), 140.5 (C-17), 141.3 (C-7a), 142.8 (C-5), 165.6 (C-2), 174.6 (C-4); FAB-Mass (m/z): 671 [M+]; Anal. Calcd. for C27H21N5O4SBr2: C, 48.30, H, 3.15, N, 10.43 %; found C, 48.23, H, 3.13, N, 10.42 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(4-nitrophenyl)-4-oxo-5-(4-nitro benzylidene)-1,3-thiazolidine-carboxamide (5h). Yield: 66%; m.p. 170-171 ΊC; IR (cm-1): 673 (C-S-C), 895 (C-N), 1351 (N-CH2), 1544 (NO2), 1567 (C=CH), 1675 (C=O), 1747 (CO cyclic), 1461, 2855, 2911 (CH2), 2952 (C=CH), 3043 (CH-Ar), 3370 (NH); 1H NMR: 1H NMR (CDCl3, 300 MHz) δ: 2.21-2.25 (m, 2H, H-9), 3.50-3.54 (m, 2H, H-10), 4.31 (t, 2H, J = 7.50 Hz, H-8), 5.23 (s, 1H, H-2), 5.82 (s, 1H, H-1), 6.64 (s, 1H, H-17), 7.86-8.46 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 36.3 (C-9), 44.3 (C-10), 50.5 (C-8), 59.5 (C-2), 120.2 (C-4), 126.7 (C-7), 128.4 (C-5), 130.1 (C-19 and C-23), 131.6 (C-12 and C-16), 132.2 (C-3a), 132.7 (C-20 and C-22), 133.5 (C-14), 134.5 (C-13 and C-15), 135.3 (C-18), 137.4 (C-6), 139.3 (C-3), 139.8 (C-11), 141.5 (C-17), 142.4 (C-7a), 144.7 (C-5), 164.2 (C-2), 174.4 (C-4); FAB-Mass (m/z): 603 [M+]; Anal. Calcd. for C27H21N7O8S: C, 53.72, H, 3.50, N, 16.24 %; found C, 53.69, H, 3.49, N, 16.20 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(3-nitro phenyl)-4-oxo-5-(3-nitro benzylidene)-1,3-thiazolidine-carboxamide (5i). Yield: 65%; m.p. 173-175 ΊC; IR (cm-1): 675 (C-S-C), 896 (C-N), 1349 (N-CH2), 1546 (NO2), 1571 (C=CH), 1676 (C=O), 1745 (CO cyclic), 1460, 2856, 2912 (CH2), 2943 (C=CH), 3045 (CH-Ar), 3369 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.26-2.30 (m, 2H, H-9), 3.54-3.59 (m, 2H, H-10), 4.28 (t, 2H, J = 7.40 Hz, H-8), 5.26 (s, 1H, H-2), 5.75 (s, 1H, H-1), 6.71 (s, 1H, H-17), 7.82-8.45 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 34.4 (C-9), 43.5 (C-10), 52.6 (C-8), 58.2 (C-2), 122.4 (C-4), 124.9 (C-7), 126.7 (C-5), 131.6 (C-19), 131.9 (C-23), 132.2 (C-12), 132.9 (C-16), 133.4 (C-20), 133.7 (C-3a), 133.9 (C-22), 134.1 (C-14), 135.0 (C-13), 135.7 (C-15), 136.9 (C-18), 138.8 (C-6), 139.5 (C-3), 140.2 (C-11), 141.4 (C-17), 141.3 (C-7a), 143.5 (C-5), 165.7 (C-2), 172.1 (C-4); FAB-Mass (m/z): 603 [M+]; Anal. Calcd. for C27H21N7O8S: C, 53.72, H, 3.50, N, 16.24 %; found C, 53.67, H, 3.47, N, 16.22 %.
N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(2-nitro phenyl)-4-oxo-5-(2-nitro benzylidene)-1,3-thiazolidine-carboxamide (5j). Yield: 64%; m.p. 172-173 ΊC; IR (cm-1): 681 (C-S-C), 897 (C-N), 1352 (N-CH2), 1543 (NO2), 1569 (C=CH), 1677 (C=O), 1749 (CO cyclic), 1458, 2858, 2914 (CH2), 2950 (C=CH), 3048 (CH-Ar), 3374 (NH); 1H NMR (CDCl3, 300 MHz) δ: 2.33-2.37 (m, 2H, H-9), 3.51-3.56 (m, 2H, H-10), 4.23 (t, 2H, J = 7.40 Hz, H-8), 5.21 (s, 1H, H-2), 5.72 (s, 1H, H-1), 6.59 (s, 1H, H-17), 7.78-8.41 (m, 12H, Ar-H); 13C NMR (CDCl3, 75 MHz) δ: 35.4 (C-9), 42.5 (C-10), 52.6 (C-8), 58.6 (C-2), 120.6 (C-4), 125.8 (C-7), 127.4 (C-5), 131.1 (C-19), 131.6 (C-23), 132.6 (C-12), 132.9 (C-16), 133.2 (C-3a), 133.7 (C-20), 133.9 (C-22), 134.6 (C-14), 135.4 (C-13), 135.8 (C-15), 136.4 (C-18), 138.5 (C-6), 140.2 (C-3), 141.7 (C-11), 140.3 (C-17), 141.8 (C-7a), 144.7 (C-5), 163.3 (C-2), 173.5 (C-4); FAB-Mass (m/z): 603 [M+]; Anal. Calcd. for C27H21N7O8S: C, 53.72, H, 3.50, N, 16.24 %; found C, 53.70, H, 3.42, N, 16.18 %.

Scheme 1. Synthesis of compound 1, 2, 3 (a-j), 4(a-j) and 5(a-j)
Biological Study
Antibacterial, Antifungal and Antitubercular Activities
Series of newly synthesized compounds were active against selected microorganisms. The minimal inhibition concentrations were determined using the filter paper disc diffusion method and the concentrations have been used in μg/mL. All the final synthesized compounds 5(a-j) have been screened in vitro for their antibacterial activity against B. subtilis, E. coli, S. aureus and K. pneumoniae and antifungal activity against A. niger, A. flavus, C. albicans and F. oxisporium Standards for antibacterial and antifungal activities Streptomycin and Griseofulvin respectively were used. The antitubercular activity screened against the M. tuberculosis. For the antitubercular activity Isoniazid and Rifampicin were used as standard. Standards also screened under the similar conditions for comparison. Results are given in Table 1 and 2.
The MIC values of standard streptomycin for all bacteria strain and Griseofulvin for all fungi strain were in the range of 1.25-3.25 and 6.25-12.5 μg/ml respectively.
Isoniazid and Rifampicin were used as standards. MIC values of 1.25 and 2.50 μg/mL against M. tuberculosis.
Table 1. Antibacterial and antifungal activities of compounds 5(a-j)
|
|
Antibacterial activity |
Antifungal activity |
||||||
|
Comp. |
B. subtilis |
E. coli |
S. aureus |
K. pneumoniae |
A. niger |
A. flavus |
F. oxisporium |
C. albicans |
|
5a |
12.5 |
7.25 |
12.5 |
6.25 |
15.5 |
15.5 |
18.25 |
17.50 |
|
5b |
3.75 |
6.25 |
3.25 |
3.50 |
8.5 |
10.25 |
12.25 |
12.75 |
|
5c |
6.25 |
3.25 |
6.25 |
3.25 |
10.50 |
12.75 |
12.50 |
12.75 |
|
5d |
3.50 |
6.25 |
3.25 |
6.25 |
13.5 |
12.25 |
14.50 |
13.75 |
|
5e |
6.25 |
3.75 |
3.25 |
4.25 |
11.50 |
12.75 |
13.50 |
13.25 |
|
5f |
6.25 |
3.25 |
6.25 |
3.25 |
12.50 |
15.5 |
10.75 |
12.50 |
|
5g |
3.50 |
6.25 |
3.75 |
6.25 |
8.25 |
14.5 |
13.25 |
14.50 |
|
5h |
3.25 |
4.25 |
3.25 |
3.25 |
9.0 |
13.50 |
11.50 |
13.25 |
|
5i |
3.25 |
3.25 |
3.50 |
3.25 |
10.50 |
12.75 |
12.50 |
13.75 |
|
5j |
3.25 |
3.75 |
3.25 |
3.25 |
12.50 |
13.50 |
10.50 |
13.75 |
|
Streptomycin |
2.25 |
3.0 |
3.25 |
2.75 |
- |
- |
- |
- |
|
Griseofulvin |
- |
- |
- |
- |
6.25 |
8.75 |
9.25 |
12.50 |
Table 2. Antitubercular activity of compounds 5(a-j)
|
Compound |
Concentration |
Compound |
Concentration |
|
5a |
8.25 |
5f |
2.25 |
|
5b |
2.75 |
5g |
6.25 |
|
5c |
2.50 |
5h |
2.50 |
|
5d |
2.25 |
5i |
2.75 |
|
5e |
2.50 |
5j |
3.25 |
Table 3. Antiinflammatory activity of compounds 5(a-j)
|
Compound Code |
Before carageenan administration (mean ± SEM) |
Total increase in paw volume after 5 hours (mean ± SEM) |
Percent inhibition |
|
5a |
0.60 ± 0.02 |
0.16 ± 0.02 |
50.00 |
|
5b |
0.64 ± 0.02 |
0.14 ± 0.02 |
56.25 |
|
5c |
0.66 ± 0.02 |
0.13 ± 0.01 |
59.38 |
|
5d |
0.68 ± 0.02 |
0.13 ± 0.02 |
59.38 |
|
5e |
0.66 ± 0.03 |
0.14 ± 0.02 |
56.25 |
|
5f |
0.65 ± 0.02 |
0.12 ± 0.01 |
62.50 |
|
5g |
0.67 ± 0.02 |
0.13 ± 0.01 |
59.38 |
|
5h |
0.64 ± 0.03 |
0.12 ± 0.01 |
62.50 |
|
5i |
0.65 ± 0.02 |
0.10 ± 0.03 |
68.75 |
|
5j |
0.67 ± 0.03 |
0.11 ± 0.02 |
65.63 |
|
Control |
0.66 ± 0.02 |
0.32 ± 0.01 |
- |
|
Standard phenylbutazone |
0.68 ± 0.03 |
0.08 ± 0.02 |
75.00 |
Antiinflammatory Activities
Carageenan induced rat paw oedema method was employed for evaluating the antiinflammatory activity of compounds at a dose 50 mg/ kg bw in albino rats (weighing 80-110 gm, each group contain 5 animal) using phenylbutazone as a standard drug for comparison at a dose 30 mg/ kg bw. The rate paw oedema was produced by the method of winter et al. The percentage inhibition of inflammation was calculated by applying Newbould formula. In vivo study has been approved institutional ethical committee, Dr. H.S. Gour University, Sagar. Results of compounds 5(a-j) were given in Table 3.
Results and Discussion
The reaction of 1-bromo-3-chloropropane with 6-nitroindazole was carried out in the methanol to afford compound 1. The spectroscopic analyses of compound 1 showed absorption peaks for N-CH and C-Cl at 1326 cm-1 and 768 cm-1 respectively in the IR spectrum. This fact also supported by the disappearance of NH absorption of the 6-nitroindazole. The compound 1 on the reaction with urea yielded compound 2. In the spectroscopic analyses of compound 2 we found three absorption peaks in IR spectrum for NH, NH2 and CO at 3342, 3456 and 1648 cm-1 respectively while absorption of C-Cl has been disappeared in the IR spectrum of compound 1. This fact was also supported by 1H and 13C NMR spectra because two signals appeared in the 1H NMR spectrum for NH and NH2 at δ 5.72 and δ 5.92 ppm respectively. The formation of the compound 2 was fully supported by a CO group gives a signal at δ 161.7 ppm in the 13C NMR spectrum of the compound 2. All the facts together are strong evidence for the synthesis of compound 2. Compound 2 give the condensation reaction with substituted benzaldehydes to yield compounds 3(a-j). Structure confirmed by IR, 1H NMR and 13C NMR spectra of compounds 3(a-j). In the IR spectra an absorption found in the range of 1555-1580 cm-1 while a strong signal appeared in the range of δ 7.89-7.98 and δ 150.6-154.6 ppm in the 1H NMR and 13C NMR spectra of compounds 3(a-j) respectively. The facts also supported by the disappearance of the signal of NH2 in the 1H NMR spectra. The compounds 3(a-j) on reaction with thioglycolic acid in the presence of ZnCl2 gives the cycloaddition reaction and produced a five membered cyclic ring known as thiazolidinone ring, compounds 4(a-j). The compounds 4(a-j) showed a characteristic absorption of the cyclic carbonyl group in the range of 1737-1750 cm-1 in the IR spectra. The 1H NMR spectra aroused our attention and clearly indicate the presence of the active methylene group in the thiazolidine ring in the range of δ 3.28-3.40 ppm. The 13C NMR spectra of compounds 4(a-j) also supported the fact that cyclic carbonyl group present and a signal appeared in the range of δ 169.8-175.7 ppm. These all fact also supported by the two evidences that are (a) disappearance of N=CH proton and (b) appearance of N-CH proton in the range of δ 5.19-5.41 ppm in the 1H NMR spectra of compounds 4(a-j). The compounds 4(a-j) underwent the Knoevenagel condensation reaction with substituted benzaldehydes in the presence of C2H5ONa to afford the compounds 5(a-j). In the 1H NMR spectra of the compounds 5(a-j), we found the disappearance of two methylene protons of compounds 4(a-j) and an appearance of a new signal for C=CH in the range of δ 6.52-6.71 ppm in the 1H NMR and two new signals for C=CH and C=CH appeared in the range of δ 138.2-141.5 and δ 140.7-144.7 ppm in the 13C NMR spectra of the compounds 5(a-j). These all above facts clearly confirmed the synthesis of all final products.
The results of the all described activities (antibacterial, antifungal, antitubercular and antiinflammatory) were summarized in Tables 1, 2 and 3. The results of the antimicrobial screening data revealed that all the compounds 5(a-j) showed considerable and varied activity against the selected microorganisms. A new series of N-[3-(1H-6-nitroindazol-1-yl)-propyl]-2-(substituted phenyl)-4-oxo-5-(substituted benzylidene)-1,3-thiazolidine-carboxamide, compounds 5(a-j) were synthesized and screened for their antimicrobial, antitubercular and anti-inflammatory activities data (as shown in Table 1, 2 and 3) revealed that all the synthesized compounds 5(a-j) have a structure activity relationship (SAR) because activities of compounds varies with substitution. Nitro group containing compounds (5h, 5i and 5j) showed higher activity than chloro (5c, 5d), or bromo group containing compounds (5e, 5f). Chloro and bromo derivatives also have higher activity than other rested compounds. On the basis of SAR, concluded that the activity of compounds depends on electron withdrawing nature of the substituted groups. The sequence of the activity is following
NO2 > Cl > Br > OH > OCH3 > CH3
The investigation of antimicrobial (antibacterial, antifungal and antitubercular) data revealed that the compounds (5c), (5d), (5e), (5f), (5h), (5i) and (5j) displayed high activity in the series, the compounds (5b) and (5g) showed moderate activity and rest compounds showed less activity against all the strains compared with standard drugs. In the anti-inflammatory activity compounds (5c), (5d), (5e), (5f), (5h), (5i) and (5j) showed high activity while rested compounds displayed moderate to less activity.
Conclusions
In this study a new series of compounds synthesis and characterized, gave satisfactory results. Synthesized compounds screened for their biological study which displayed moderate to good activity.
Acknowledgements
The authors are thankful to SAIF, Central Drugs Research Institute, Lucknow (India) for providing spectral and analytical data of the compounds. We are also thankful to Head, Department of Chemistry, Dr. H. S. Gour, University (A Central University), Sagar (India) for giving the facilities to carry out the work.
References
1. Berhe S., Slupe A., Luster C., Jr.Henry A. C., Warner D. L., Zalkow L. H., Burgess E. M., Enwerem N. M., Bakare O., Synthesis of 3-[(N-carboalkoxy)ethylamino]-indazole-dione derivatives and their biological activities on human liver carbonyl reductase, Bioorganic & Medicinal Chemistry, 2010, 18, p. 134-141.
2. Woods K.W., Fischer J.P., Claiborne A., Li T., Thomas S.A., Zhu Gui-Dong, Diebold R.B., Liu X., Shi Yan, Klinghofer V., Han E.K., Guan R., Magnone S.R., Johnson E.F., Bouska J.J., Olson A.M., Jong R. de, Oltersdorf T., Luo Y., Rosenberg S.H., Girandaa V. L., Lia Q., Synthesis and SAR of indazole-pyridine based protein kinase B/Akt inhibitors, Bioorganic & Medicinal Chemistry, 2006, 14, p. 6832-6846.
3. Pinna G.A., Pirisi M.A., Mussinu Jean-Mario, Murineddu G., Loriga G., Pau A., Grella G.E., Chromophore-modified bis-benzo[g]indole carboxamides: synthesis and antiproliferative activity of bis-benzo[g]indazole-3-carboxamides and related dimmers, ll Farmaco, 2003, 58, p. 749-763.
4. Cottyn B., Acher F., Ramassamy B., Alvey L., Lepoivre M., Frapart Y., Stuehr D., Mansuy D., Boucher J-L, Vichard D., Inhibitory effects of a series of 7-substituted-indazoles toward nitric oxide synthases: Particular potency of 1H-indazole-7-carbonitrile, Bioorganic & Medicinal Chemistry, 2008, 16, p. 59625973
5. Doris M., Ulf G., Carsten T., Hans F., 7-Nitro indazole enhances methohexital anesthesia, Brain Research, 1998 78, P. 353-355.
6. Gerpe A., Aguirre G., Boiani L., Cerecetto H., Gonzalez M., Olea-Azar C., Rigol C., Maya J. D., Morello A., Piro O. E., Aran V. J., Azqueta A., Cerain A. Lopez de, Monge A., Rojas M. A.,Yaluff G., Synthesis, biological evaluation and mechanism of action studies, Bioorganic & Medicinal Chemistry, 2006, 14, p. 3467-3480.
7. Xua X., Qianb X., Zhong L., Synthesis and fungicidal activity of fluorine-containing phenylimino-thiazolidines derivatives, Journal of Fluorine Chemistry, 2005, 126, p. 297-300.
8. Li W., Lu Y., Wang Zhao., James T., Dalton, Miller D. D., Synthesis and antiproliferative activity of thiazolidine analogs for melanoma, Bioorganic & Medicinal Chemistry Letters, 2007, 17, p. 4113-4117.
9. Vazzana I., Terranovaa E., Mattiolib F., Sparatorea F, Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents, Arkivok, 2004, 5, 364-374.
10. Orrling K. M., Marzahn M. R., Gutierrez-de-Teran H., Aqvist J., Dunn B. M., Larhed M., A-Substituted norstatines as the transition-state mimic in inhibitors of multiple digestive vacuole malaria aspartic proteases, Bioorganic & Medicinal Chemistry, 2009, 17, 5933-5949.
11. Li G., Qian X., Cui J., Huang Q., Cui D., Zhang R., Liu F., Synthesis and herbicidal activities of fluorine-containing 3-pyridylmethyl-2-phenyliminothiazolidine derivatives, Journal of Fluorine Chemistry, 2006, 127, p. 182-186.
12. Ami E., Nakahara K., Sato A., Nguyen J.-T., Hidaka K., Hamada Y., Nakatani S., Kimura T., Hayashia Y., Kisoa Y., Synthesis and antiviral property of allophenylnorstatine-based HIV protease inhibitors incorporating D-cysteine derivatives as P2/P3 moieties, Bioorganic & Medicinal Chemistry Letters, 2007, 17, p. 4213-4217.