Effect of Firing Temperature on Mechanical Properties of Fired Masonry Bricks Produced from Ipetumodu Clay
Fatai Olufemi ARAMIDE
Department of Metallurgical and Materials Engineering, Federal University of Technology, P.M.B. 704, Akure, Nigeria.
E-mail: fat2003net@yahoo.com or foaramide@futa.edu.ng
Abstract
The effect of varied firing temperature on the mechanical properties of fired masonry bricks samples produced from Ipetumodu clay was investigated. The clay sample was characterized using scanning electron microscopy (SEM) for the evaluation of the morphology of the sample using secondary electron imaging; and the phases/compositions of the samples using energy-dispersive X-ray (EDX) analysis, X-ray diffractometer (XRD), X-ray fluorescence (XRF) and atomic absorption spectroscopy (AAS). The brick samples of standard dimensions were prepared from the clay slurry. The prepared samples were sun dried for 72 hours and then fired at varied temperature (held for an hour) and then allowed to cool to room temperature in the furnace. The mechanical properties (compression strength, shear strength, modulus of rupture, density and hardness) of the samples were then investigated. It was observed that the mechanical properties of the fired brick samples varied with varying firing temperature due to phase changes/chemical reaction between the phases in the clay sample. It was concluded that the optimum mechanical property for brick samples within the temperature range considered is obtained at 950oC.
Keywords
Masonry bricks; Firing temperature; Modulus of rupture; Shear strength.
Introduction
Clays are vital industrial raw materials, they are utilized in the fields of ceramics, paper, paint, petroleum industry, catalysis etc. [1, 2]. Their applications are highly dependent upon their structure, composition, and physical attributes [3]. The knowledge of these characteristics can help for a best exploitation and eventually may open-up new areas of application. Clays are widely used in the manufacture of many traditional ceramics. Each ceramic product requires clays having particular and appropriate characteristics, they must not contain a swelling phase, their loss of weight and shrinkage, after drying firing, have to be low [4]. Clay bodies undergo several changes during drying and firing stages as a result of physical, chemical and mineralogical modifications. Kaolinite-illite is the most widely used clay mixture in the ceramic industry. Illite is one of the main clay phases used for the preparation of mixtures for traditional ceramics. The knowledge of the mineralogical phase composition of the raw materials used for the preparation of ceramic mixtures is of principal importance for understanding of the technological properties of ceramic products and optimizing of firing cycles in production.
Clays are widely used in vitreous ceramic and construction industries [5-7] and are generally mixed and blended to give standard compositions and optimum properties for various applications [8]. The microstructure and properties of clays depend on the zaacharacteristics of the parent clay and the processing conditions [9]. Various types of clays comprise different clay minerals of the ninety-three planar/non-planar hydrous phyllosilicates [10, 11] which are either 1:1 or 2:1 layer type phyllosilicates with/without interlayer water [12].
Primarily, the native people of the Ipetumodu town of the south western part of Nigeria utilize the enormous deposit of clay the nature endowed them with for pottery purposes. Various researchers had investigated the properties of burnt bricks made from other clay deposit; for instance, [13] examined properties of raw materials and burnt bricks produced from Nigerian soils such as the utilization of lateritic clays at Ala River, Akure and Okeigbo near Ondo, Nigeria. He obtained high crushing strength values for bricks made from the different varieties of clay and found them to be suitable for bricks and ceramics industries in view of their good quality. Other researchers [14, 15] also obtained similar results for the clay deposits in parts of south western Nigeria.
Even though Ibitoye and Afonja, [16, 17] had established the possibility of adapting these natural resources for foundry purposes; there have been virtually little/no information on the mechanical properties of the fired bricks produced from this clay deposit for masonry purposes. The availability of this information can lead to industrial revolution in the area and impart positively on the socio-economic life of the native resident. This research endeavour is aimed and directed toward this end.
Materials and Methods
The materials used for this study include; Ipetumodu clay as mined, water, wooden sieve, beaker, conical flask and thermometer.The equipment used for this study include; a furnace, mounting press, grinding mill, pulverizer, seive shaker and set of sieves, and compression strength tester.
Clay acquired from Ipetumodu, Osun State was soaked in water for three days to dissolve the clay and at the same time to form slurry. The resulting slurry was then sieved to remove dirt and other foreign substances using a sieve. More water was thereafter poured into the clay to form slurry once again. This is then allowed to settle down for seven days. The floating clear liquid was decanted after the seventh day. The settled fine clay was then poured into a P.O.P mould and left undisturbed for three days in other to allow the liquid still present to drain out completely. The resulting plastic clay was then rammed into mould of standard masonry brick dimension. The prepared samples were sun dried for 72 hours and then placed in the furnace and fired at various temperatures of 750oC (A), 800oC (B), 850oC (C), 900oC (D) and 950oC (E); held at the temperature for 1 hour. Series of tests were then performed on the fired samples. These tests include; cold crushing strength, bulk density, modulus of rupture, shear strength and hardness (Brinell hardness).
Shear Testing
The specimens were loaded with shear stress on a Mosanto Tensometer until failure. The loading is applied by means of compression force only. But due to the inclination of the bed joints which are at an angle with the loading direction, the sample is loaded by a combination of normal compression and shear. From the applied normal stress and the angle of inclination of the bed joints with the loading direction, the shear stress is calculated [18]. A graph of the applied stress against extension for each specimen was simultaneously plotted by the machine.
Compressive Testing
The specimens were loaded with uniaxial compressive load on a Mosanto Tensometer until failure. A graph of the applied stress against extension for each specimen was simultaneously plotted by the machine [19].
Modulus of Rupture Test
Moduli of rupture tests were performed on a standard mechanical machine. Test specimens, of each of the brick composition were dried and fired at the appropriate firing temperature as earlier mentioned. Each of them was placed one after the other on the bearing edges of the compression machine positioned 7.0 cm apart. Loads were then applied at the middle of the specimens, uniformly at 1.25 Kgf per minute. Modulus of rupture (R), Kgf/cm2, calculated from the relation given by [20]: R = (3wl)/(2vd2), were w = total load at which the specimen failed, Kg; l = distance between support, that is bearing edges, cm; b = width of specimen, cm; d = depth of specimen, that is thickness, cm.
Brinell Hardness Indenter Test
An indenter was pressed into each sample by an accurately controlled test force. The force was maintained for a dwell time of about 10 - 15 seconds, after which the indenter was removed leaving a round indent in the sample. The size of the indent was determined optically by measuring two diagonals of the round indent. The Brinell hardness number is a function of the test force divided by the curved surface area of the indent. The average of the two diagonals was used to calculate the Brinell hardness in the formula [21]
![]()
Bulk Dnsity
The test specimens were dried at 110ºC for 12 hours to ensure total water loss. Their dry weights were measured and recorded. They were allowed to cool and then immersed in a beaker of water. Bubbles were observed as the pores in the specimens were filled with water. Their soaked weights were measured and recorded. They were then suspended in a beaker one after the other using a sling and their respective suspended weights were measured and recorded. Bulk densities of the samples were calculated using the formula:
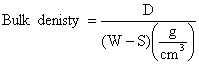
were: D = Weight of dried specimen, S =Weight of dried specimen suspended in water, and W = Weight of soaked specimen suspended in air.
Qualitative and Quantitative XRD
The samples were prepared for XRD analysis using a back loading preparation method. They were analysed using a PANalytical X’Pert Pro powder diffractometer with X’Celerator detector and variable divergence- and receiving slits with Fe filtered Co-Ká radiation. The phases were identified using X’Pert Highscore plus software. Graphical representations of the qualitative result follow below.
The relative phase amounts (weight %) were estimated using the Rietveld method (Autoquan Program). Amorphous phases, if present were not taken into consideration in the quantification. The quantitative results are listed below. The data generated is shown in Figure 1.0 and Table 2.0.
Scanning Electron Microscopy
Morphology and microanalysis of the clay samples were determined by SEM/EDX. The pulverized clay samples were previously graphite coated. The clay samples were studied with a Philips® 30 Analytical Scanning Electron Microscope. Particle images were obtained with a secondary electron detector. The SEM/EDX micrograph of the powder clay sample is show in Figure 1.0.
Chemical Analysis
The major elements were determined by X-ray fluorescence with an ARL® 9800 XP spectrometer. The pulverized clay samples were mixed with lithium tetraborate for chemical analysis. The ignition loss was measured by calcinations at 1000 °C. The XRF result is shown in Table 3.
Results and Discussion
Tables 1, 2 and 3 shows respectively the atomic absorption spectroscopy result, the XRD result showing the quantity of phases present and XRF semi-quantitative analysis of the raw clay sample.
Table 1. Atomic Absorption Spectroscopy Result of Clay Sample (%)
|
Samples |
SiO2 |
Al2O3 |
Fe2O3 |
TiO2 |
CaO |
MgO |
Na2O |
K2O |
LOI |
|
Raw Clay |
63.42 |
32.58 |
2.62 |
1.00 |
0.12 |
0.10 |
0.04 |
0.09 |
11.26 |
Figures 1 shows the XRD pattern of the clay sample while Figure 2 shows the SEM/EDX micrograph of the raw clay sample.
Furthermore, Figures 3 to 7 respectively shows the effect of firing temperatures on the density, modulus of rupture, Brinell hardness number (BHN), shearing strength and the compression strength of the fired brick samples.
Table 2. XRD Result of the Raw Clay Samples Showing the Quantity of Different Phases Present
|
Phases |
Weight % |
|
Kaolinite |
23.74 |
|
Microcline |
26.12 |
|
Muscovite/Illite |
15.02 |
|
Plagioclase Albite |
11.28 |
|
Quartz |
23.84 |
Table 3. XRF Semi-quantitative analysis of the elements of raw clay samples (weight %)
|
Phases |
Al2O3 |
SiO2 |
Fe2O3 |
K2O |
MgO |
Ba |
CaO |
Cl |
Co |
Cr2O3 |
|
Weight % |
25.03 |
57.48 |
9.226 |
1.26 |
0.91 |
0.08 |
0.76 |
0.02 |
0.02 |
0.045 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Phases |
Cu |
MnO |
Na2O |
Ni |
P2O5 |
SO3 |
Sr |
TiO2 |
Zr |
Total |
|
Weight % |
0.03 |
0.05 |
0.37 |
0.08 |
0.05 |
0.03 |
0.02 |
1.51 |
0.1 |
97.1 |
From Table 2, it can be observed that the feldspar content of the clay sample is very high; it contains 26.12% microcline and 11.28% plagioclase/albite, it also contain 15.02 muscovite/illite (micas). This agrees with the report of [22] on Reference soil moist lowlands near Ile-Ife. Feldspar is an important industrial material used in the production of glass, and other sintered ceramics. This is because it favours liquid phase formation and densification at low temperature [23, 24].
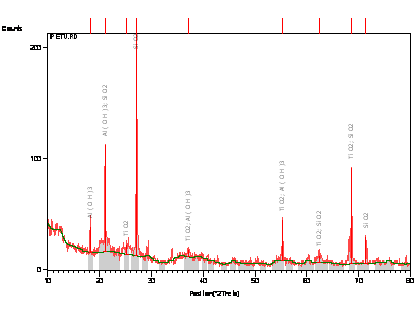
Figure 1. X-Ray Diffraction Pattern (Phase Analysis) of the Clay Sample
Generally speaking, the clay mineralogical content comprises of kaolinite, feldspars (microcline and plagioclase/albite), muscovite/illite, (micas) and quartz. From Table 2 and 3 it can be seen that the clay have high alumina content; from minerals point of view, the clay could be subjected to appropriate concentrating technique to produce high grade alumina for various industrial applications. Moreover the clay is a potential raw material for porcelain and glassmaking industries.
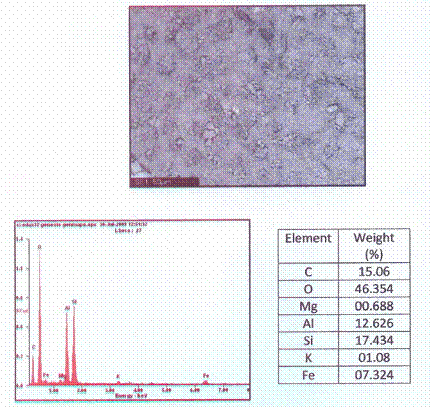
Figure 2. A Typical Scanning Electron/EDX Micrograph of the Clay Sample
From Figure 3, it is observed that the density of the fired brick samples initially increased with increased firing temperature from 750oC to 800oC and then the density decreased with further increase in firing temperature until it reached the minimum density (at 950oC) for the considered firing temperatures. The explanation for this is that transformation of clays from one phase to another or from one type of clay to another type is a function of temperature, pressure, time, contact with microbes and chemicals, the type and quantity of impurities, and exposure to radiations [25]. Clays may be thermally transformed into other phases when subjected to heating. This process is accompanied by weight loss and sometimes conservation of the residual structurally bound water into the next phase [26]. The dehydration of kaolinite (99% pure English china clay) completes by ~150oC, followed by dehydoxylation at ~500-600oC and its structural breakdown occurs in the temperature range ~800-900oC, depending upon the particle size and amount and type of the impurities present [27, 28 and 29].
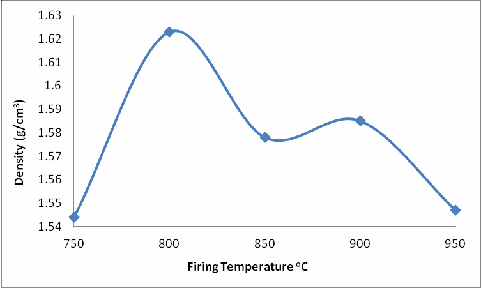
Figure 3. Effect of Firing Temperature of the Bulk Density of the Brick
Clay from Ipetumodu (Table 2) containing 23.84% quartz, 15.02% muscovite/illite (and about 37% feldspars) if feldspars were not involved dehydroxylates to metakaolinite at ~420-660°C depending on the type and amount of impurities present [30] via
![]()
which involves the combination of two OH groups to form H2O and oxygen which remains incorporated in metakolin. At about 900°C, metakaolinite decomposes to amorphous SiO2 and ã-Al2O3–type spinel via
![]()
ã-Al2O3–type spinel and SiO2 re-crystallize into mullite at temperatures above 1100°C via
![]()
Sonuparlak et al (1987) reported the observation of a ã-Al2O3–type spinel phase along with some residual amorphous SiO2 while Srikrishna and his colleagues [31] supported the formation of a single phase (with composition close to mullite) and excess SiO2 at 900°C. Thus the formation of mullite begins at temperatures >900°C and the process continues till 1000°C.
The case of the sample concerned of more than binary phase, the presence of feldspar and kaolinite together with the quartz and muscovite/illite make phases involved to be more complex [32]; this may account for the initial increase in density of the samples as they were fired from 750oC to 800oC
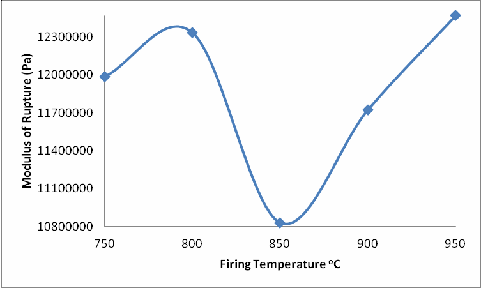
Figure 4. Effect of Firing Temperature on the Modulus of Rupture of the Brick
From Figure 4, it is observed that the modulus of rupture of the fired bricks similarly increased with increase in firing temperature from 750oC to 800oC and then reduced with further increase in the firing temperature from 800oC to 850oC, and then increased with subsequent increase in the firing temperatures. The explanation for this is, due to the phase changes (and reactions) that occurred in the samples during firing, cracks were initiated within the brick samples (fired at 850oC); these cracks serves as weak point within the body of the brick samples hence the reduction in the modulus of rupture of the sample fired at 850oC when compared with that of the sample fired at 800oC. Above 850oC (900oC) liquid phase sintering (due to the presence of feldspar) sets in and began to be filling up the cracks thereby strengthening the sample, accounts for the increase in the modulus of rupture of the fired bricks (fired at 900oC and above).
The same explanation could be given for Figures 6 and 7 with a little difference in Figure 6 at 900oC. Because it is shear stress and the glassy phase has not fully filled up some of the remaining cracks, that is why the shear strength at 900oC is lower than at 800oC.
From Figure 5 the effect of firing temperatures on the Brinell harness of the fired brick sample is clearly depicted; it is observed that the hardness of the samples initially increased slightly from samples fired at the 750oC through to those one fired at 850oC, and then reduced to the minimum.
For samples fired at 900oC, further increase in the firing temperature resulted in sharp increase in the Brinell hardness of the samples fired at 950oC. This could be attributed to presence of the glassy phase and the formation of mullite in the sample fired at 950oC
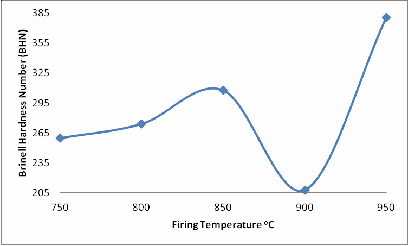
Figure 5. Effect of Firing Temperature on the Brinell Hardness Number of the Brick
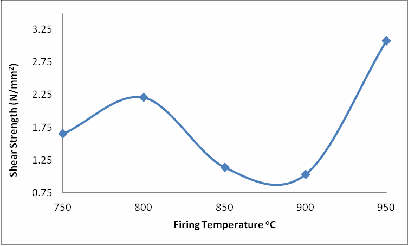
Figure 6. Effect of Firing Temperature on the Shear Strength of the Brick
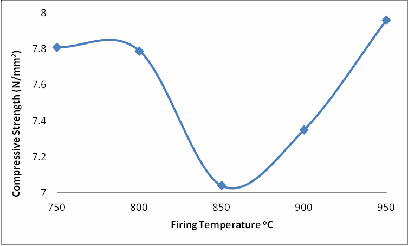
Figure 7. Effect of Firing Temperature on the Compressive Strength of the Brick
Conclusion
From the discussion so far it is concluded that:
§ The mechanical properties of the fired brick samples varied with varying firing temperature due to phase changes/chemical reaction between the phases in the clay sample.
§ The optimum mechanical property for brick samples within the temperature range considered is obtained at 950oC
References
1. Mortland M., Shaobai S., Boyd S., Clayorganic complexes as adsorbants for phenols and chlorophenols, Clays and Clay Minerals, 1986.
2. Burch R., Warburton C., Pillared Clay as Demetallisation Catalysts, Applied catalyse, 1987.
3. Grim R. Some applications of clay mineralogy, American Mineralogist, 1960.
4. Aydin A. The change of phase composition in kaolinite- and illite-rich Clay- based ceramic bodies, Applied Clay Science, 2004.
5. Grim R.E., Objectives of the first conference on clays and clay technology and definitions of terms used in the industry, Clays Clay Miner, 1952, 1, p. 13-15.
6. Murray H.H., Traditional and new applications for Kaolinite, Smectite, and palygorskite: a general overview, Appl. Clay Sci., 2000, 17, p. 207-221.
7. Temimi M., Amor K.B., Camps J.P., Making building products by extrusion and cement stabilization: limits of the process with montmorillonite clays, Appl. Clay Sci., 1998, 13, p. 245-253.
8. Bailey R.T., The changing pattern of raw material supplies, J. Brit. Ceram. Soc., 1974, LXXIII, p. 283-286.
9. Dananaj I., Frankovska J., Janotka I., The influence of smectite content on microstructure and geochemical properties of calcium and sodium bentonites, Appl. Clay Sci., 2005, 28, p. 223-232.
10. Bergaya F., Theng B.K.G., Lagaly G., Development in Clay Science, Handbook of Clay Science, Elsevier Ltd., 2006.
11. Guggenheim S., Adams J.M., Bain D.C., Bargaya F., Brigatti M.F., Drits V.A., Formoso M.L.L., Galan E., Kogure T., Stanjek H., Summary of the recommendations of nomenclature committees relevant to clay mineralogy: report of the Association International pour l’Etude Argiles (AIPEA), Nomenclature Committee for 2006, Clay Minerals, 2006, 41, p. 863-877.
12. Bailey S.W., Summary of recommendation of AIPEA nomenclature committee on clay minerals, Am. Mineral., 1980, 65, p. 1-7.
13. Mesida E.A., Utilization of some Lateritic Clays for Burnt Bricks, Journal of Mining and Geology, 1978, 15(2), p. 108-114.
14. Durotoye B., Ige A., Awojide P.O., Small scale mining of clay deposit at Ipetumodu, near Ile- Ife, Oyo State, Journal of Mining and Geology, 1988, 24(l&2), p. 65-69.
15. Ogunsawo O., CBR and strength of compacted soils from southwestern Nigeria, QJEG, 1989, 22(4), p. 317-328.
16. Ibitoye S.A., Afonja A.A, Adaptation of Ipetumodu potter’s clay to foundry use: 1. Moulding properties of as mined and silica mixed potter’s clay, Ife Journal of Technology, 1997, 7(1), p. 17-22.
17. Ibitoye S.A., Afonja A.A, Adaptation of Ipetumodu potter’s clay to foundry use: 2. Development of potter’s clay bound synthetic moulding sand, Ife Journal of Technology, 1997, 7(1), p. 39-45.
18. Monk C.B., Testing high-bond clay mansory assemblages, American Society for Testing Materials (ASTM), 1962, 320.
19. Aramide Fatai Olufemi, Production and Characterization of Porous Insulating Fired Bricks from Ifon Clay with Varied Sawdust Admixture, Journal of Minerals and Materials Characterization and Engineering, 2012, 11(10), p. 970-975.
20. Chesti A.R., Refractories: manufacture, properties, and applications, Delhi, Prentice-Hall of India Private Limited, 1986.
21. Lysaght V.E., Indentation Hardness Testing, Reinhold Publishing Corp., New York, 1949.
22. Fagbami A.A., Shogunle E.A.A., Nigeria: Reference Soil of Moist Lowlands near Ife (Osun State),. Soil Brief Nigeria 3. Univesity of Ibadan, Ibadan and International Soil Reference and Information Centre, Wageningen, 1995, p. 13.
23. Ergul S., Akyildiz M., Karamanov A., Ceramic material from basaltic tuffs, Industrial Ceramics, 2007, 27(2), p. 89-94.
24. Kingery W.D., Introduction to Ceramics, J. Wiley & Sons, New York, 1976.
25. Kim J, Dong H, Seabaugh J, Newell SW, Elbert DD., Role of Microbes in the smectite-to-Illite reaction, Science, 2004, 303, p. 830-832.
26. Lee S, King KJ, Lee HL, Moon HS., Electron-Beam-Induced phase transformations from Metakaolinite to Mullite Investigated by EF-TEM and HRTEM., J. Am. Ceram. Soc., 2001, 84, p. 2096-2099.
27. McConville C.J., Lee W.E., Microstructural development on firing illite and smectite compared with that in kaolinite, J. Am. Ceram. Soc., 2005, 88, p. 2267-2277.
28. McConville C.J., Related microstructural development on firing keolinite, illite and smectite clays, PhD thesis. University of Sheffield (UK), 1999.
29. McConville C.J., Lee W.E., Sharp J.H., Comparison of Microstructural evolution in kaolinite powders and dense clay bodies, Brit. Ceram. Trans., 1998, 58, p. 75-92.
30. Qiu G., Jiang T., Li G., Fan X., Huang Z., Activation and removal of silicon in kaolinite by thermochemical process, Scan. J. Metallurgy, 2004, 33, p. 121-128.
31. Srikrishna K., Thomas G., Martinez R., Corral M.P., Aza S.D., Moya J.S., Kaolinite-mullite reaction series: A TEM study, J. Mater. Sci., 1990, 25, p. 607-612.
32. Weill D.F., Kudo A.H., Initial melting in alkali feldspar-plagioclase-quartz systems, Geological Magazine, 1968, 105, p. 325-337.