Deposition and Characterization of Silver Oxide from Solution of Silver, Cassava and Sugarcane Juice Effects
Uche E.
Ekpunobi*, Austine
I Anozie, Onyebuchi O. OKWUKOGU, Adaora S. OGB
Pure and Industrial Chemistry Department, Nnamdi Azikiwe University, P.M.B. 5025, Awka, Anambra State. Nigeria.
E-mail: ask4uche2001@yahoo.com
* Corresponding author: +234-8037614963
Abstract
Silver oxide was deposited on metallic substrates (zinc and lead) from silver solution with different additives at a pH of 5, dc current of 0.2A, 4V for 20seconds at 25°C. The additives were cassava solution and sugarcane juice. The metallic substrates served as cathode while a copper electrode serves as the anode. Compositions of the electrolytes were 50ml AgNO3, 50ml AgNO3 and 50ml of cassava solution or 50ml of sugarcane juice. Structural and textural characterizations were carried out on the deposits. The result showed that deposition using zinc substrate gave a better result than that of lead in that the deposits were pure without impurities. Using cassava solution as additive, a pure Ag2O3 deposit was obtained while sugarcane juice gave a pure intergrowth of Ag2O3 and Ag3O4 deposits both on zinc substrates.
Keywords
Silver; Electrodeposition; X-Ray Diffraction (XRD); Characterization; Cassava juice; Sugarcane juice.
Introduction
Silver is found in nature form as an alloy with gold (electrum) and in ores containing sulphur, arsenic, antimony or chlorine [1]. The metal is primarily produced as a by-product of electrolytic copper, gold, nickel, and zinc refining and by application of the parks process on lead metal obtained from lead ores that contain small amount of silver [2]. Silver metal dissolves readily in nitric acid (HNO3) to produce nitrate (AgNO3), a transparent crystalline solid that is photosensitive and readily soluble in water. Silver nitrate is used as a starting point synthesis of many other silver compounds, as an antiseptic, as a yellow stain for glass in stained glass. Silver metal does not react with sulphuric acid which is used in jewelry-making to clean and remove copper oxide fire scale from silver articles after soldiering or annealing. Compounds of silver have found usage in many different ways. For instance, silver halides are used in manufacture of photographic emulsions [3]. Silver chlorides are used in glass-electrodes for pH testing and potentiometric measurements and as transparent cement for glass. Silver iodide has been used in attempt to seed cloud to produce rain [4]. Silver fulminate (AgONC), a powerful touch, sensitive explosive used in percussion caps [5]. Silver cyanides solutions are used in electroplating of silver [6]. Silver oxides are used in organic chemistry as mild oxidizing agent, while they are used commercially in silver oxide battery. Like many silver compound, silver oxide is photo sensitive and decomposes at temperatures above 280oC. Silver I oxide (Ag2O) is is-structural with copper I oxide (Cu2O) hence it is expected that Ag2O is insoluble in all solvents except by reaction [7]. Some electrical and electronic products use silver for its superior conductivity even when tarnished. The primary example of this is in high quality Rf connectors. Silver cadmium oxides are used in high voltage contacts because it can withstand arcing. Some manufacturers produce audio connector cables, speaker wires and power cable using silver conductors which have a 6% higher conductivity than ordinary copper one of identical dimensions but very much more. The amount of silver coated glass worldwide is hundreds of millions of square meter per year leading to silver consumption on the order of 10 cubic meters of 100 metric tons/year [8]. In this work, we studied the elctrodeposition and characterization of silver oxides from silver solution on metallic substrates. In order to improve adhesion to the substrates, increase brightness and reduce toxicity of cyanide, the use of cassava and sugarcane solutions in place of sodium cyanide was made.
Material and Method
Preparation of AgNO3 Solutions
All chemicals were purchased from the head bridge market, Onitsha. 0.5M of silver nitrate AgNO3 was prepared by dissolving 10g of AgNO3 in a 500ml of distilled water. 1M solution of sodium cyanide was prepared by dissolving 25g of sodium cyanide in 500ml of distilled water.
Electrodeposition
Electrodeposition was carried out in three baths. The composition of the electrolyte in the first bath is 10ml AgNO3 solution and 50ml NaCN. Zinc substrate was used as the cathode while copper electrode was used as the anode. Electrodeposition was carried out for 20seconds under 4volts. The deposition was repeated using lead substrate in place of zinc. The pH of solution was 9.0.
Electrodeposition with Cassava Solution
In another bath, all materials as in the first bath above were added except sodium cyanide which was now replaced by addition of 50ml of saturated solution of mashed fresh cassava tuber [9]. Electrodeposition was also carried out as in first bath above. The pH of solution was 5.9
Electrodeposition with Sugarcane Solution
The experiment was repeated in third bath using 50ml of undiluted sugarcane juice in place of sodium cyanide, the cathode and anode remaining the same as in the first bath. The pH of solution was 5.1
At the end of electrodeposition, the coated substrates were washed well with distilled water and air dried at room temperature. The thickness of the films produced was determined gravimetrically. Appearance of the films deposited from the baths was recorded. Electrodeposition was done at room temperature (26°C)
Characterization
The dried substrates were taken for topographical and structural characterization using photomicrograph (x 60 magnification) and XRD (x-ray minidiffractometer (10) with CuKα radiation and excitation energy of 25KV) manufactured by Radicon ltd respectively.
Results and Discussion
Structural Characterization
Figs.1a and 1b show the x-ray diffraction spectra of silver oxide thin films prepared from AgNO3 and NaCN on zinc and lead metallic substrates respectively.
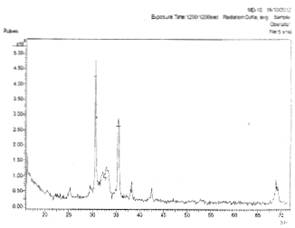
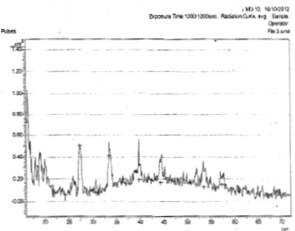
Figure 1a (left) and 1b (right). X-ray diffraction spectra from AgNO3 and NaCN solution on zinc and lead metallic substrates respectively
In Fig. 1a, there is (111), (200), and (222) planes of reflection of siver oxide (Ag2O) film while others were impurities. In Fig. 1b, there is intergrowth of Ag3O4 and Ag2O3 shown in planes (110), (032) and (202), (311), (800) respectively. Others were impurities. Figs. 2a and 2ba and 2b show the x-ray diffraction spectra of silver oxide thin films prepared from AgNO3 and cassava solution on zinc and lead metallic substrates respectively. In Fig. 2a, there is only one plane (031) of reflection of 100 intensity of monoclinic silver oxide (Ag3O4) film.
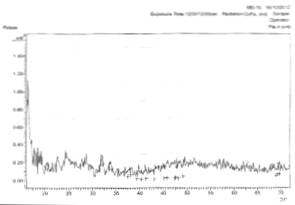
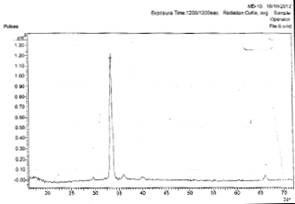
Figure 2a (left) and 2b (right). X-ray diffraction spectra from AgNO3 and cassava solution on zinc and lead metallic substrates respectively
In Fig. 2b, there was no prominent peak depicting presence of much impurities. However a plane of (200) reflection of Ag2O is shown.
Figs. 3a and 3b show the x-ray diffraction spectra of silver oxide thin films prepared from AgNO3 and sugarcane juice solution on zinc and lead metallic substrates respectively.
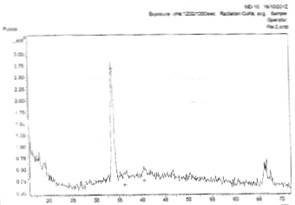
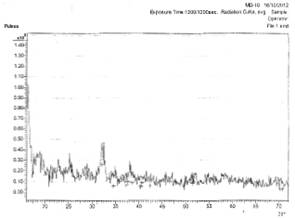
Figure 3a (left) and 3b (right). X-ray diffraction spectra from AgNO3 and sugarcane solution on zinc and lead metallic substrates respectively
In Fig. 3a, there is planes of relections (031), (1-12), and (171) of Ag3O4, Ag, and Ag2O3 film respectively.
Topographical Characterization
The topographical characterization of the films deposited is in good agreement with the XRD result (Figures 4-6). All deposition on zinc substrates were seen to be more smooth, homogenous, dense and has a clear image view than those on lead substrate.


Figure 4a (left) and 4b (right). Micrograph of the film deposited from AgNO3 and NaCN solution on zinc and lead metallic substrates respectively


Figure 5a (left) and 5b (right). Micrograph of the film deposited from AgNO3 and cssava solution on zinc and lead metallic substrates respectively
From the results it is shown that lead metallic substrate, there were much impurities introduced in the thin film whereas zinc metallic substrate gave a better deposition with less impurity. There is a great effect on addition cassava and sugarcane juice solutions. Both enhanced the deposition ability. It is also deduced that cassava solution gave a better deposition with high purity than sugarcane juice solution.


Figure 6a (left) and 6b (right). Micrograph of the film deposited from AgNO3 and sugarcane juice solution on zinc and lead metallic substrates respectively
Conclusion
Silver oxides were successfully deposited on the metallic substrates. The use of cassava and sugarcane solutions in improved adhesion to the substrates, increase brightness and reduced toxicity of cyanide. The films deposited on zinc metallic substrate gave pure silver oxide thin films than that on lead substrate. Depositions with cassava solution gave a better film with high purity than sugarcane juice solution.
Acknowledgements
We acknowledge the assistance of the XRD Department of Energy Centre Obafemi Awolowo University, Ile-Ife in characterization of the materials.
References
1. Hammond C. R., The Elements, in handbook of Chemistry and Physics 81st edition. CRC Press, 2000.
2. The Silver Institute (Online). Silver Supply & Demand. Thomson Reuters. 36-241
http://www.silver institute.org/supply demand.php. Retrieved 2012-03-29.
3. Blelkhagen H. I., Silver-halide recording materials for holography and their processing. Springer. ISBN 3-540-540-58619-9, 1995, p. 156-166.
4. The
Silver Institute (Online). World Silver Survey 2011.
5. Meyer R, Kohler J., Homburg A., Explosives, Wiley - VCH, ISBN 3-537-31656-6, 2007, p. 284.
6. CPM
Group. CPM Silver yearbook,
7. Dirkse T. P., Copper, Silver, Gold and Zinc, Cadmium, Mercury Oxides and Hydroxides
Pergamon, Oxford, 1986.
8. Merck
E. Merck index of chemicals and drugs,Merck and Co. Inc.
9. Ajiwe V. I. E., Anyadiegwu I. E., Recovery of silver from industrial wastes, cassava solution effects, Separation and Purification Technology, 2000, 18(2), p. 89-92.