Removal of Heavy Metals Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+ from Aqueous Solutions by using Xanthium Pensylvanicum
Jaber SALEHZADEH
Department of Chemistry Ardabil Branch, Islamic Azad University, Ardabil City, Iran
E-mail: chem.salehzadeh@yahoo.com
Corresponding author: Phone: +98 9144524649; Fax: +98 4517717382
Abstract
The hazardous ill effects of heavy metals on the environment and public health is a matter of serious concern. Biosorption is emerging as a sustainable effective technology. Heavy metals in water resources are one of the most important environmental problems of countries. The intensification of industrial activity and environmental stress greatly contributes to the significant rise of heavy metal pollution in water resources making threats on terrestrial and aquatic life. The toxicity of metal pollution is slow and interminable, as these metal ions are non bio-degradable. The adsorption capacity of Xanthium Pensylvanicum towards metal ions such as Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+, was studied. The adsorption capacity was performed by batch experiments as a function of process parameters (such as sorption time and pH). Experimental results showed that the removal percentages increasing of metal ions at pH=4, initial concentration of metal ions 10 mg/L, and after 90 min of shaking was: Zn2+ < Cd2+ < Cu2+ < Pb2+ < Ni2+ < Fe3+ < Co2+.
Keywords
Xanthium Pensylvanicum; Heavy metals; Removal of metals; Absorption.
Introduction
Heavy metals in water resources are one of the most important environmental problems of countries. Due to modernization, the industrial use of metals especially, heavy metals, has risen alarmingly, thus becoming of prior concern because of their toxicity to flora and fauna. Recovery of heavy metals from industrial waste streams is becoming increasingly important as society realizes the necessity for recycling and conservation of essential metals [1]. The intensification of industrial activity and environmental stress greatly contributes to the significant rise of heavy metal pollution in water resources making threats on terrestrial and aquatic life. The toxicity of metal pollution is slow and interminable, as these metal ions are non bio-degradable. Until now, various methods of removing of these metals have been considered, including the use of low price materials [2-6]. The use of natural materials for heavy metal removal has become a concern in all countries. Natural materials that are available in large quantities or certain waste from agricultural processes may have potential as low cost adsorbents, as they represent unused resources, widely available and are environmentally friendly [7]. To see the decrease of polluted water, most researches were concentrated with treatment of heavy metals from industrial wastewater. This uses normal material to removal metals from different sides because it is valid largely in agriculture processes in addition to their low price as adsorbent materials [8].
Activated sludge is used as bioadsorbent for Cu2+, Cd2+ and Ni2+. Pretreatment with NaOH was found to improve the adsorption capacity of the sludge, whereas treatment with HCl reduces it [9]. Anaerobic sludge supplied from a wastewater treatment plant, acts as a novel biosorbent, for Pb2+, Cu2+, Cd2+, and Ni2+ removal from aqueous solutions [10]. Rice husk, a surplus agricultural byproduct, is used for the sorption of Cd2+ from aqueous solution. Some simple and low-cost chemical modifications resulted in increasing the sorption capacity of raw rice husk (RRH) has been studied [11]. Papaya wood was evaluated as a new biosorbent of heavy metal ions such as Cu2+, Cd2+ and Zn2+ [12]. The sorption of lead, copper, cadmium, zinc and nickel by marine algal and characterization of biosorptive capacity were significantly affected by solution pH [13]. Coffee residues binding with clay as adsorbent (hereafter called CC-adsorbent) are utilized for removal of heavy metal ions in solution [14]. Cocoa shells (CS) have been identified as a very efficient natural sorbent to remove Pb2+ and other metal ions from acid soil leachates (ASL) [15]. Fungal biomass immobilized within a loofa sponge (FBILS) is used as a new biosorbent system to remove heavy metal ions such as Pb2+, Cu2+ and Zn2+ from aqueous solution [16]. Calcined phosphate is a good adsorbent for the removal of Pb2+, Cu2+, and Zn2+ from solutions. The abundance of natural phosphate, its low price and non-aggressive nature towards the environment are advantages for its utilization in the point of view of wastewater and wastes clean up [17].
This study aims to use cheap materials like Xanthium Pensylvanicum to remove the heavy metal ions from waste water instead of the classical techniques that are difficulty controlled, and requiring mostly expensive equipments.
Material and Method
The adsorbent material Xanthium Pensylvanicum was collected from Parsabad Moghan region in Ardabil province and after being dried was cut into small pieces of size5 mm. The plant particles were washed with tap water followed by deionized water, then filtered and finally dried at 65 ºC for 24 hours. They were homogenized to mesh 100 in a mortar and bolter and subsequently used for adsorption experiments. Adsorption studies were performed by batch experiments as a function of process parameters (such as sorption time and pH). Freundlich model fitted best with the experimental equilibrium data among the all tasted adsorption isotherm models.
Experimental Reagents and Apparatus
Reagents used were lead, copper, zinc, cadmium, nickel, cobalt, and iron standard solutions 1000 ppm (Merck), sodium hydroxide (Merck), nitric acid (Merck). A Varian (240AA) flame atomic absorption spectrophotometer (air/acetylene flame) was used for metal ions determinations. A Metrohm (model 780) digital pH meter equipped with a combined glass electrode was used for the pH adjustments. IKA stirer and centrifuge were also used.
Preparation of Synthetic Wastewater
The mixed metal ions solution from cations(Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+) was prepared from Merck – analytical grade stock standard of concentration 1000 ppm. The solution of wastewater was introduced for each metal according to the method of continuous dilution from the sample solution earlier mentioned. The pH of the wastewater was adjusted by using HNO3 1% (V/V) and/or NaOH. The final concentration of metal ions in wastewater was analyzed by atomic absorption spectrophotometer type Varian – 240AA.
Estimation of Removal Percentage of Metal Ions by Plant Materials
Amount of removed material by plants through series of batch investigations were determined by the following equation: Removal %= [(Co - Cf)/ Co] × 100, where Co and Cf are the initial and equilibrium concentration (ppm) of metal ions in solution, respectively.
Results and Discussion
Effect of Weight of Xanthium Pensylvanicumon: the Removal of the Heavy Metal Ions
Multi-element standard solution containing (Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+) metal ions which concentration was equal to 10 mg/L, was prepared. The pH of the standard solution was adjusted to 4. To 50 mL of the multi-element standard, 0.1, 0.3 and 0.6gr of the Xanthium Pensylvanicum were added in an Erlenmeyer flask, and the mixtures were shaken using a rotary shaker at about 200 rpm for 90 min. After that the mixtures were filtered using a Whatman no. 1 filter paper. The filtrate and the multi-element standard were analyzed using an atomic absorption spectrophotometer. Each experiment was carried out at room temperature and was repeated two times, and the results are given as averages. The effect of weight of sorbent Xanthium Pensylvanicum plant on the percent removal of Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+and Fe3+ is shown graphically in Figure 1. Inspection of the data obtained showed that:
1. Maximum percent removal was obtained for Co2+ and Fe3+ ions, which is nearly equal to 98% and 97.9 %, respectively, but it decreased very slightly by increasing the weight of sorbent.
2. Minimum removal was obtained for Zn2+ ion, which is slightly increased by the increase in the weight of sorbent.
3. The variation of maximum percent removal of metal ions with weight of Xanthium Pensylvanicum used as sorbent lies in the order Co2+>Fe3+>Ni2+>pb2+>Cu2+>Cd2+>Zn2+.
4.
Effect of Contact Time on the Removal of the Heavy Metal Ions
A multi-element standard solution was prepared. To 50 mL of each solution, 0.3 gr of the Xanthium Pensylvanicum were added and the mixtures were shaken for 30, 60, 90 and 120 minutes, and analyzed using an atomic absorption spectrophotometer. Adsorption Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+ ions were measured at given contact times for initial metal ions concentrations of 10 mg/L. The plot revealed that the rate of percent metal ions removal is higher at the beginning. This was probably due to larger surface area of the plants being available at beginning for the adsorption of Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+ ions. As the surface adsorption sites become exhausted, the uptake rate was controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles. Most of the maximum percent Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+ removal was attained after about 90 min of shaking time. Results of studies on the effect of contact time on the maximum removal of the metal ions under investigation, illustrated in Figure 2.
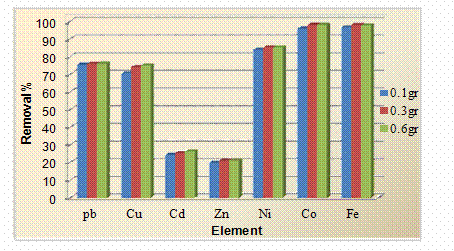
Figure 1. Effect of weight of Xanthium Pensylvanicumon the removal of heavy metal ions
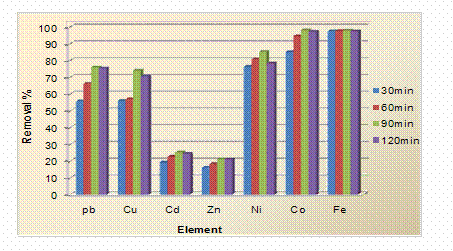
Figure 2. Effect of contact time on the removal of heavy metal ions
Effect of pH on the Removal of the Heavy Metal Ions
The adsorption of Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+ ions were found to be strongly dependent on the pH of the solution and analyzed using atomic absorption spectrophotometer. It was demonstrated that the optimum pH for the adsorption metal ions were about 4 which were rather acidic. At low pH (below 3), there was excessive protonation of the active sites at Xanthium Pensylvanicum powder surface and this often refuses the formation of links between metal ions and the active site. At moderate pH values (3 to 6), linked H+ is released from the active sites and adsorbed amount of metal ions is generally found to increase (Figure 3). Moreover, at higher pH values (above 6), the precipitation was dominant or both ion exchange and aqueous metal hydroxide formation may become significant mechanisms in the metal removal process. In practice, metal precipitation is generally not a stabilized form of heavy metal as the precipitation can sometimes be very small in size, and upon the neutralization of the effluent from the wastewater treatment plant, the solubility of the metals increases resulting in a recontamination of the waste outlet stream.
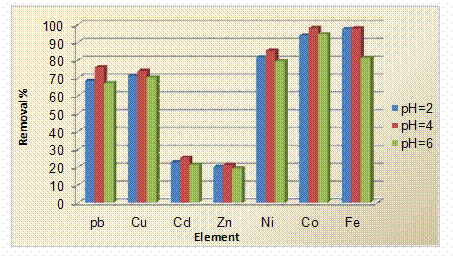
Figure 3. Effect of pH on the removal of heavy metal ions
Effect of Initial Concentration of the Heavy Metal Ions on the Removal Percentage
A series of multi-element standard solutions with concentrations of 5, 10, 30 and 50 mg/L were prepared. The solutions were treated as previously and analyzed using atomic absorption spectrophotometer. The variation of percent removal with change in initial concentration of heavy metal ions showed regular trend. the percent removal for metal ions increases with increasing the initial concentration, till reaching 10 mg/L, then decreases as the initial concentration increases up to 50 mg/L. The results are present in Figure 4.
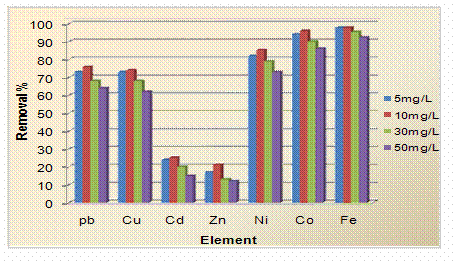
Figure 4. Effect of initial concentration of the heavy metal ions on the removal percentage
Conclusions
Based on the present investigation, it could be concluded that some low cost materials like xanthium pensylvanicumcan can be used efficiently in the removal of heavy metal ions (Pb2+, Cu2+, Zn2+, Cd2+, Ni2+, Co2+ and Fe3+) from aqueous solutions. The removal of heavy metal ions was pH dependent as the adsorption capacity increases with increasing the pH value of the solution, and at a particular pH the order of increasing the removal percentage was Zn2+< Cd2+< Cu2+<pb2+< Ni2+< Fe3+< Co2+. Experimental results showed that the best pH for adsorption was 4 and time was 90 min. The metal ions showed different behaviors towards adsorption on Xanthium Pensylvanicum by increasing the initial concentration of the metal ions. This investigation also showed absorbent prepared from Xanthium Pensylvanicum to be suitable adsorbent for removing the Co2+ and Fe3+ heavy metal ions. Adsorption of some metal ions was fitted with Langmuir isotherm, other ones with Freundlich isotherm, and the others were fitted with both the models. The experimental studies showed that Xanthium Pensylvanicum could be used as an alternative, inexpensive and effective material to remove high amounts of toxic heavy metal ions from wastewater.
References
1. Jothinayagi N., Anbazhagan C., Heavy Metal Monitoring of Rameshwaram Coast by some Sargassum species, American-Eurasian J. Sci. Res, 2009, 4, p. 73-80.
2. Shafaghat A., Salimi F., Valiei M., Salehzadeh J., Shafaghat M., Removal of heavy metals (Pb2+, Cu2+ and Cr3+) from aqueous solutions using five plants materials, African J. Biotechnology, 2012, 11(4), p. 852-855.
3. Devaprasath P. M., Solomon J. S., Thomas B. V., Removal of Chromium (VI) from Aqueous Solutions using Natural Plant Mater, J. Appl. Sci. Environ. Sanitation, 2007, 2, p. 77-83.
4. Al-Subu M. M., Salim R., Abu-Shqair I., Swaileh K. M., Removal of dissolved copper from polluted water using plant leaves: I. Effects of acidity and plant species, Rev. Int. Contam. Ambient, 2001, 17, p. 91-96.
5. Mehrasbi M. R., Farahmand Kia Z., Heavy Metal Removal from Aqueous Solution by Adsorption on Modified Banana Shell Iran, J. Health . Environ, 2008, 1, p. 57- 66.
6. Uzun I., Guzel F., Adsorption of Some Heavy Metal Ions from Aqueous Solution by Activated Carbon and Comparison of Percent Adsorption Results of Activated Carbon with those of Some Other Adsorbents, Turk. J. Chem, 2000, 24, p. 291- 297.
7. Afkhami A., Madrakin T., Karimi Z., Effect of the impregnation of carbon with ethylenediamintetraaceticacid on its adsorption capacity for the adsorption of several metals ions, J. Hazard. Mater, 2008, 150, p. 408- 412.
8. Deans J. R., Dixon B. G., Uptake of Pb and Cu by novel biopolymers, Water Res, 1992, 26(4), p. 469-472.
9. Al-Qoda Z., Biosorption of heavy metal ions from aqueous solutions by activated sludge, Desalination, 2006, 196, p. 164-176.
10. Hawari H. A., Mulligan N. C., Biosorption of lead (II), cadmium (II), copper (II) and nickel (II) by anaerobic granular biomass, Biores. Tech, 2006, 97, p. 692-700.
11. Kumar U., Bandyopadhyay M., Sorption of cadmium from aqueous solution using pretreated rice husk, Biores. Tech, 2006, 97, p. 104-109.
12. Saeed A., Akhter M. W., Iqbal M., Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent, Sep. &Purifi. Tech, 2005, 45, p. 25-31.
13. Sheng P. X., Ting Y. P., Chen J. P., Hong L., Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms, J. Colloid. Interface Science, 2004, 275, p. 131-141.
14. Boonamnuayvitaya V., Chaiya C., Tanthapanichakoon W., Jarudilokkul S., Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay, Sep. & Purifi. Tech, 2004, 35, p. 11-22.
15. Meunier N., Blais J. F., Tyagi R. D., Removal of heavy metals from acid soil leachate using cocoa shells in a batch counter-current sorption process, Hydrometallurgy, 2004, 73, p. 225-235.
16. Iqbal M., Edyvean R. G. J., Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaetechrysosporium, Min. Eng, 2004, 17, 217-223.
17. Aklil A., Mouflih M., Sebti S., Removal of heavy metal ions from water by using calcined phosphate as a new adsorbent, J. Hazard. Materials, 2004, 112, 183-190.