Optimization of the recycle used oil and its fuel quality characterization
Eyitayo A. AFOLABI*, Abdulsalam S. KOVO, Olalekan, D ADENIYI, Abdulsalam JIBRIN, Ambali S. ABDULKARREM, Bilyaminu SULEIMAM
Department of Chemical Engineering, School of Engineering and Engineering Technology,
Federal University of Technology, PMB 65, Gidan Kwano, Minna, Nigeria
* Corresponding author: elizamos2001@yahoo.com
Abstract
The optimization of recycling of used engine oil with clay sample has been studied using Response Surface Methodology. Acid concentration, activation temperature and time were the independent variables considered in optimizing the recycling of used oil and six responses evaluated. The surface characterization of the clay samples was performed using the Fourier Transform Infrared (FTIR) spectra and Brunauer Emmett Teller (BET) analyses. The relationship between independent variables and response was described by a second order polynomial equation. Statistical testing of the model was performed with F-test to obtain the correlation between the experimental data and predicted results for all responses. The adequacy of the model equations were evaluated by the Adjusted and Predicted R2 coefficients observed to be close to each other for all the six responses. Data obtained from recycling used oil using clay sample showed the optimum condition as; activation temperature of 106.80oC, acid concentration of 3M and activation time of 180 minutes. A yield of 66.28% was obtained at optimum condition and characterized fuel qualities found close to fresh oil used as standard in this work. The surface area and adsorption capacity of raw clay and activated clay samples was observed to have increase from 19.8m2/g to 437.83m2/g and 1.41 mg/g to 8.64 mg/g respectively. This difference adequately described the improvement of the adsorption phenomena of the activated clay over raw clay samples.
Keywords
Optimization; Clay samples; Used engine oil; Recycling; Response Surface Method (RSM)
Introduction
Motor oil is used for lubricating various internal combustion engines to reduce friction, protect against wear and tear and helps in distribution of heat. During normal use, many impurities such as soot, water, acids, dirt, metal scrapings, and chemicals can get mixed in the oil, and becomes ineffective for further application [1,2]. The current disposal practice of just dumping used oils in drains, rivers and lagoons poses serious environmental and health hazards. Thus, it is imperative to find more environmentally sustainable ways to dispose used lubricating oils through recycling. Recycling of the waste lubricating oil is now regarded as the most viable option to address the environmental dangers posed by the indiscriminate waste engine oil disposal. In addition, recycling is also considered as an effective means of conserving the depleting world crude reserve and job creation through the construction and operation of recycling plants [3,4]. Several methods of recycling waste lubricating oil have evolved over time from the period which recycling of waste oil began during the late 1930s. These methods include; acid method, distillation/clay method, acid/clay method, activated charcoal/clay method among others. Since the inception of waste lube oil recycling, clay has been one of the most important and widely used adsorbent materials for the renewal of the oil [5]. The clay/acid method was the earliest method employed in recycling used engine oil and observed to have high adsorption capacity to remove most of the metallic buildup in the spent engine oil [6].
The improved property achieved through acid modification of clays is now generating a renewed interest by researchers in recycling waste engine oil [4, 6]. Recycling of waste lubricating oil ensure reduction of the environmental menace posed by the indiscriminate waste lube oil disposal. In addition, it serves as the most reliable mean of regenerating the useful hydrocarbon which can be further processed and re-use. The aim of this study was to investigate the fuel quality of used lube oil recycled through the clay samples activated under varying conditions of time, concentration and temperature using the Response Surface Methodology. Further objectives include determination of the optimum conditions necessary for activation of clay samples; recycling of used oil with activated clay samples; calculation of the adsorption capacity of the treated clay; characterization of recycled lube oil samples and comparison with standard lubricating oil obtained in the market.
Material and method
The clay used in this work was collected from a clay deposit, Ahoko near Lokoja, Kogi State. The used oil was a Total Quartz 5000 20W-50, collected from a local mechanic workshop, Minna, Nigeria. The glass wares used in this work were provided by the Chemical Engineering Department Laboratory of Federal University of Technology, Minna.
Experimental design
A Central Composite Design (CCD) of Response Surface Methodology (RSM) was used to design the optimization of treated clay in the recycling of used engine oil. In this study, independent variables considered are activation temperature, activation time and acid concentration. Based on CCD for five-levels with three variables as shown in Tables 1 and 2, a total of 19 experimental runs comprise of 8 factorial points, 6 axial points and 5 central points were carried out [7]. The optimum values from the 3×3 factorial design work carried out by Yahaya [8] was used as a basis for the optimization at Zero level in this work
Table 1. Independent variables and natural levels used for central composite design
|
Variable |
Symbol |
Coded factor level |
||||
|
-1.68 |
-1 |
0 |
1 |
1.68 |
||
|
Activation temperature |
A |
73.20 |
80.00 |
90.00 |
100.00 |
106.80 |
|
Acid concentration |
B |
2.16 |
2.50 |
3.00 |
3.50 |
3.84 |
|
Activation time |
C |
154.80 |
165.00 |
180.00 |
195.00 |
205.20 |
Clay preparation
The untreated clay sample was grounded and mixed with distilled water to form a solution. Impurities such as sand, stone and other particles are allowed to settle at the bottom of the cylinder and then separated by decanting off the clay particles. The slurry was kept in an oven to dry up. The dried clay was thereafter grounded and sieved into very fine particle size using 75 µm mesh size sieve.
Clay activation
One hundred (100) grams of raw clay sample was weighed using a digital weighing balance into a double neck round bottom flask and 250 ml of 2.5 mol/dm3 H2SO4 solution added. A reflux condenser was connected to the flask at one neck and a thermometer inserted at the other neck of the flask. The bottom and top openings of the condenser were connected to a source of running cold water and a sink respectively. The setup was clamped firmly to the magnetic stirrer operating at 800 rpm, temperature of 80oC for 165 minutes. The same procedure was repeated in order to generate the remaining 18 experimental runs as shown in Table 2.
Purification process of the used engine oil
The spent engine oil was purified using the treated clay samples. The percolation method of treatment as described by Grim [9] was employed in the purification of the oil. The spent oil was preheated for about 10 minutes to lower it viscosity and increase it penetration power. 30 g of the treated clay samples, each were packed in a plug flow funnel (funnel inserted with a filter paper). Then after, 80 ml of the preheated spent oil was measured and poured over the clay bed in the funnel and allowed to filter through the bed into a conical flask.
Characterization of clay samples
The surface area of the raw and activated clay samples were determined experimentally using the Quantachrome Nova 5200, Brunauer – Emmett –Teller (BET) surface analyzer. The moisture content and bulk density of the clay were determined according to the procedure discussed by Dada et al [10]. Fourier Transform Infrared (FTIR) spectra analysis of the raw and activated clay samples was done according to ASTM model FTIR 84005 methods in order to have better insight into the functional groups available on the surface of the clay samples [11].
Characterization of oil
The fresh, used and recycled oil samples were analyzed using Atomic absorption spectrophotometer for iron content. The flash point, specific gravity, viscosity and cloud point of the oil samples were determined according to the ASTM D97 procedure as discussed by Udonne [6]. Yield, a very important factor that reflects the effectiveness of the optimization was calculated according to Equation 1.
|
|
(1) |
Experimental optimization
A statistical program package, Design Expert 7.0.0, was used for regression analysis of the data obtained and to estimate the coefficients of the regression equation. The equations were validated by the statistical tests called the analysis of variance (ANOVA).
Adsorption capacity of clay
The adsorption capacity of the treated clay was calculated according to Equation 2 (Al-Khatib et al. [12]);
|
|
(2) |
where q1= adsorption capacity of the raw clay; q2= adsorption capacity of the activated clay (mg metal/g of the adsorbent); v= total volume of oil used (mL); Ci= Initial concentration of the metal in the oil (mg/L); Cf= final concentration of the metal in the oil (mg/L); m= amount of Activated clay (g)
Results and Discussion
The characterization results for treated used lube oil with activated clay and statistical analysis of the responses from RSM are showed in Tables 2 to 4. Table 5 showed the BET analysis of the raw clay and clay samples activated at optimum condition. Table 6 summarizes the fuel qualities of fresh lube oil, used oil and treated used oil at optimum condition. The comparison of the experiment data with predicted results for responses is illustrated by Figure 1. Figures 2 and 3 are FT IR analysis of raw and activated clay samples.
Table 2. Characterization results of treated used oil
|
S/N |
Point type |
Temp (oC) |
Conc. (M) |
Time (Minute) |
Viscosity (mm2/s) |
Flash point (oC) |
Cloud point (oC) |
Specific gravity |
Iron content (ppm) |
Yield (%) |
|
1 |
Factorial |
80.00 |
2.50 |
165.00 |
19.5 |
91.00 |
12.69 |
0.895 |
0.55 |
59.50 |
|
2 |
Factorial |
100.00 |
2.50 |
165.00 |
20.0 |
97.00 |
12.80 |
0.893 |
0.35 |
62.83 |
|
3 |
Factorial |
80.00 |
3.50 |
165.00 |
20.5 |
91.30 |
12.74 |
0.894 |
0.60 |
61.77 |
|
4 |
Factorial |
100.00 |
3.50 |
165.00 |
20.0 |
98.00 |
13.56 |
0.892 |
0.30 |
65.10 |
|
5 |
Factorial |
80.00 |
2.50 |
195.00 |
18.5 |
101.00 |
14.00 |
0.885 |
0.76 |
62.50 |
|
6 |
Factorial |
100.00 |
2.50 |
195.00 |
17.5 |
107.60 |
14.20 |
0.878 |
0.35 |
63.50 |
|
7 |
Factorial |
80.00 |
3.50 |
195.00 |
18.5 |
101.33 |
14.58 |
0.883 |
0.60 |
64.77 |
|
8 |
Factorial |
100.00 |
3.50 |
195.00 |
16.0 |
108.00 |
14.80 |
0.873 |
0.30 |
66.10 |
|
9 |
Axial |
73.18 |
3.00 |
180.00 |
20.5 |
93.90 |
13.09 |
0.894 |
0.80 |
60.32 |
|
10 |
Axial |
106.80 |
3.00 |
180.00 |
18.0 |
105.10 |
14.86 |
0.881 |
0.30 |
66.28 |
|
11 |
Axial |
90.00 |
2.16 |
180.00 |
20.0 |
99.22 |
12.96 |
0.891 |
0.50 |
62.55 |
|
12 |
Axial |
90.00 |
3.84 |
180.00 |
19.5 |
99.78 |
13.04 |
0.887 |
0.40 |
65.50 |
|
13 |
Axial |
90.00 |
3.00 |
154.77 |
20.5 |
91.10 |
12.70 |
0.895 |
0.42 |
60.60 |
|
14 |
Axial |
90.00 |
3.00 |
205.23 |
17.0 |
107.90 |
15.00 |
0.876 |
0.48 |
65.00 |
|
15 |
Center |
90.00 |
3.00 |
180.00 |
19.0 |
99.50 |
14.00 |
0.888 |
0.42 |
64.50 |
|
16 |
Center |
90.00 |
3.00 |
180.00 |
19.0 |
99.50 |
14.60 |
0.889 |
0.44 |
63.50 |
|
17 |
Center |
90.00 |
3.00 |
180.00 |
19.5 |
99.30 |
14.50 |
0.887 |
0.45 |
63.50 |
|
18 |
Center |
90.00 |
3.00 |
180.00 |
19.0 |
99.40 |
14.40 |
0.888 |
0.46 |
63.50 |
|
19 |
Center |
90.00 |
3.00 |
180.00 |
19.5 |
99.50 |
14.50 |
0.888 |
0.45 |
64.80 |
Table 3. Analysis of variance (ANOVA) on velocity
|
Source squares |
Sum of squares |
df |
Mean Prob. >F |
F |
p-value |
|
Model |
26.09 |
9 |
2.90 |
19.23 |
<0.0001 |
|
A-Temperature |
4.35 |
1 |
4.35 |
28.83 |
0.0005 |
|
B-Conc. |
0.13 |
1 |
0.13 |
0.87 |
0.3744 |
|
C-Time |
17.33 |
1 |
17.33 |
114.99 |
<0.0001 |
|
AB |
0.78 |
1 |
0.78 |
5.18 |
0.0488 |
|
AC |
1.53 |
1 |
1.53 |
10.16 |
0.0111 |
|
BC |
0.78 |
1 |
0.78 |
5.18 |
0.0488 |
|
A2 |
0.079 |
1 |
0.079 |
0.53 |
0.4865 |
|
B2 |
0.14 |
1 |
0.14 |
0.91 |
0.3638 |
|
C2 |
0.87 |
1 |
0.87 |
5.80 |
0.0394 |
|
Residual |
1.36 |
9 |
0.15 |
|
|
|
Lack of Fit |
1.06 |
5 |
0.21 |
2.82 |
0.1686 |
|
Pure Error |
0.30 |
4 |
0.075 |
|
|
|
Cor. Total |
27.45 |
18 |
|
|
|
Statistical analysis of the model
The relationship between the independent variables and response (Viscosity) is described by a second order polynomial equation given as:
Viscosity=19.22-0.56*A-0.098*B-1.13*C-0.31*A*B-0.44*A*C-0.31*B*C-0.076*A2+0.1*B2-0.25*C2
The statistical significance of the model equation was performed with F-Test ANOVA, and the result obtained shown in Table 3. The F-value of 19.23 with P-value less than 0.0001 indicates that the model equation is significant and able to predict viscosity as a response by 99.99 % accuracy. For a model term in design expert analysis to be considered significant, the P- value must be less than 0.05 [5]. As shown in Table 4, model terms A, C, AB, AC, BC, C2 were found significant and B, A2 and B2 insignificant.
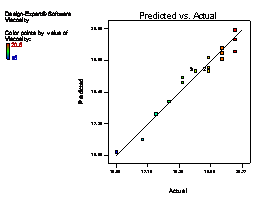
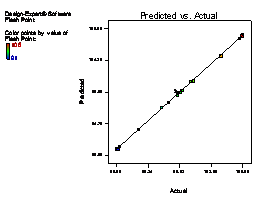
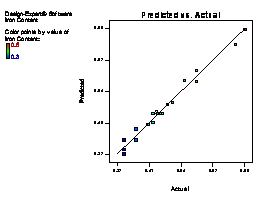
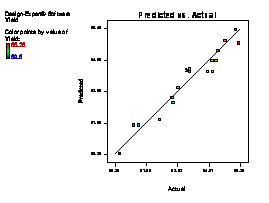
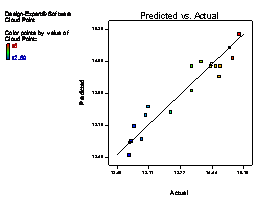
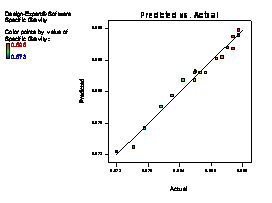
The model terms A, B, C, AB, AC, BC, A2 and C2 are terms with negative coefficient, thereby exhibiting an inverse proportionality on the viscosity response. The fit of the model was evaluated by the coefficient of R2 calculated to be 0.9506, thereby indicating that 95.06 % of the variability in the response (viscosity) could be explained by the model. The "Lack of Fit F-value" of 2.82 implies that the Lack of Fit is not significant relative to the pure error (which is a desirable condition). This signifies that there is a minimal difference between the predicted values generated by the model equation and the experimental data. Figure 1(a) showed that a linear relationship exist between the actual and predicted values, thereby indicating that the model equation adequately predict the viscosity within the limit of experimental condition investigated. The same pattern was observed in Figure 1 (b-f) for the comparison between experimental data and predicted results for flash point, iron content, yield, cloud point and specific gravity responses respectively.
Table 4. R2 statistics for the regression models
|
Response |
R2 |
Adjusted R2 |
Predicted R2 |
Adequate precision |
|
Viscosity |
0.9506 |
0.9011 |
0.6898 |
16.443 |
|
Flash point |
0.9996 |
0.9993 |
0.9974 |
164.499 |
|
Cloud point |
0.9283 |
0.8566 |
0.5522 |
11.155 |
|
Specific gravity |
0.9808 |
0.9616 |
0.8678 |
24.016 |
|
Iron content |
0.9806 |
0.9612 |
0.8439 |
25.847 |
|
Yield |
0.9443 |
0.8885 |
0.7223 |
15.133 |
A rule of thumb suggested by Bas and Boyaci [13] is that, the adjusted and predicted R2 should be close to unity and their difference not more than 0.3. As shown in Table 4, the close relationship between the R2 of 0.901 and predicted R2 of 0.7 revealed the goodness of the model for viscosity and thereby makes the model for predicting viscosity as a response valid.
Among the 19 experiments, 4 experiments were repetition of the central point (Run 16-19). These are the experiments in which all the factors are in the centric point of values. The closeness of the responses of these 4 experiments is an evidence that the predicted results is accurate on comparison with the experimental data. Experimental run 10 was found to be the best run as it has the optimum yield of 66.28% with the flash point, cloud point, specific gravity, viscosity and iron content of the used oil is in a close agreement with that of fresh oil on comparison with other experimental runs. Therefore, conditions at which experimental run 10 was carried out is henceforth referred to as optimum conditions.
|
(a) |
(b) |
|
(c) |
(d) |
|
(e) |
(f) |
Figure 1. Plot of actual experimental value and predicted value on (a) Viscosity (b) Flash point (c) Iron Content (d) Yield (e) Cloud point (f) Specific gravity
Physico-chemical analysis of the clay samples
The physic-chemical properties of the raw, activated and spent clay samples such as moisture content, bulk density and BET surface area are given in Table 5.
Table 5. Physico-chemical parameters of the clay samples
|
Property |
Raw Clay sample |
Activated clay: optimum condition |
Spent clay |
|
Moisture content (%) |
0.5 |
0.4 |
0.47 |
|
Surface area (m2/g) |
19.8 |
437.83 |
127.09 |
|
Bulk density (g/ml) |
1.25 |
1.35 |
1.31 |
There is an increase in the BET pore surface area for raw and activated clay samples from 19.8 m2/g to 437.83 m2/g respectively. This is expected as acid activation of clay sample is an effective method in the creation of more surface area for adsorption process. This is in agreement with the work of Jabit [14], Singh et al. [15] and Al-Sultani and Al-Seroury [16]. They all observed that, the adsorptive capacity of an adsorbent depends on its surface area (i.e. the greater the surface area the higher the material’s sorption capacity). However, there is a decrease in the BET pore surface area from 437.83 to 127.09 m2/g for the activated and spent clay samples respectively. This reduction is as a result of more sites or pores created by activation that is now occupied by the contaminants after the adsorption process.
Fourier Transform Infrared (FTIR) analysis
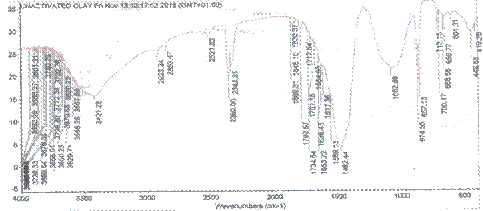
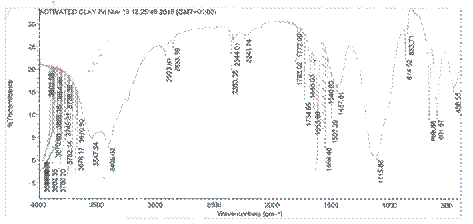
FTIR technique is used mainly to identify functional groups such as carboxyl, hydroxyl, etc. that are capable of adsorbing contaminants. Figures 2 and 3 show FTIR spectra in the 419-3840 cm-1 wave number ranges for the raw and activated clay samples. A number of absorption peaks were displayed, reflecting the complex nature of clays with some peaks observed to have shifted or disappeared and new ones detected on comparison of the FTIR analysis of raw and activated clay samples.
These changes observed in the spectra of both clay samples confirmed that activation process modified the clay sample [17]. During activation of the clay sample, protons from the acid medium penetrate into the clay structures and attack the OH- groups thereby causing the alteration in the adsorptive bands attributed to the OH vibrations and octahedral cations. The increase in the severity of acid is shown by disappearance of the stretching bands at 857, 1482 and 2360 cm-1 assigned to H-O-H stretching. The peak at 1845, 3802 and 3886 still remained after acid treatment with slight increase in the intensities. The same result was reported by Chistidis et al. [11].
|
|
|
Figure 2. FTIR spectrum of unactivated clay |
|
|
|
Figure 3. FTIR spectrum of activated clay |
Adsorption capacity
The adsorption capacity of the raw and activated clay samples was calculated to be 1.41mg/g and 8.64mg/g respectively. This implies that at optimum conditions, adsorption capacity of clays sample used in this work increases by 436.7%.
Lube oil characterization
The main characteristics of fresh oil, used oil, raw clay treated oil and optimum activated clay treated oil are presented in Table 6.
Table 6. Characteristics of fresh oil, used oil, raw clay treated oil and optimum activated clay treated oil
|
Sample |
Yield (%) |
Flash point (oC) |
Cloud point (oC) |
Specific gravity |
Viscosity Cst |
Iron content (ppm) |
|
Fresh Oil |
- |
120 |
16.50 |
0.871 |
21.90 |
0.01 |
|
Used Oil |
- |
77 |
10.00 |
0.904 |
15.20 |
3.54 |
|
Oil treated with raw clay |
40.00 |
79 |
12.00 |
0.896 |
15.90 |
3.01 |
|
Oil treated with activated clay at optimum conditions |
66.28 |
105.10 |
14.86 |
0.881 |
18.00 |
0.30 |
Yield of 66.28% was obtained when used oil is treated with activated clay sample obtained at optimum conditions against 40 % obtained for raw clay sample. Thus, the treated clay sample is observed to have adsorbed more contaminants than the raw clay sample. This confirmed that more contaminants were removed from the used oil by treated clay sample at optimum conditions than the raw clay sample. This clearly demonstrates that more surface area was created at the optimum conditions and responsible for adsorption of more contaminant.
The Flash point of fresh oil is 120oC while that of used oil was determined to be 77oC. The drop in flash point is as a result of the dilution of the lubricant with unburned fuel during engine operations [18]. The flash point of treated used oil with raw and activated clay samples were determined to be 79.0oC and 105.10oC respectively. The increase in flash point of used oil treated with activated clay at optimum conditions is due to reduction of contaminants in the lubricating oil. The flash point obtained is observed to be much closed to the flash point of fresh oil.
The cloud points of fresh and used oil were observed to be 16.50oC and 10.0oC respectively. A decrease in cloud point reflects the presence of contaminants in the used lubricating oil [5,18]. However, treating the used lubricating oil with raw clay and activated clay sample at optimum conditions showed an increase in cloud points of recycled oil from 12.0oC to 14.86oC respectively. Similarly, cloud point of 14.86oC for used oil treated at optimum a condition was observed to be close to 16.50oC for the fresh oil. This clearly confirmed the activated clay sample at optimum conditions is effective for the purification of the used lubricating oil.
As shown in Table 6, the specific gravity of fresh lubricating oil is 0.871 while that of the used oil was found to be 0.904. Udonne [6] attributed the high specific gravity of the used oil to the presence of contaminants such as metals and degraded products. However, on treatment with raw clay and activated clay sample at optimum conditions, the specific gravity decreases to 0.896 and 0.881 respectively. The specific gravity of 0.881 for oil treated with activated clay sample was observed to be closer to 0.871 for that of the fresh oil. This showed the ability of the activated clay sample to regenerate the used lubrication oil.
The viscosities of fresh and used lubricating oil were measured to be 21.90 mm2/s and 15.2mm2/s respectively. Ajemba et al., [16] suggested that a decrease in viscosity of lubricating oil is as a result of dilution with light fuel and this is observed on comparison of the viscosity of fresh oil to that of the used oil. However, viscosities of oil treated with raw and activated clay were found to be 15.90 and 18.0 respectively. Thus, activated clay was able to adsorbed more contaminants from the used oil than raw clay. This confirmed that, activation of clay sample increased the adsorption capacity of the clay as the viscosity of the oil treated with activated clay is closer to that of the fresh oil.
The iron contents of the fresh oil and that of the used lubrication oil were found to be 0.01ppm and 3.54 ppm respectively. The increase in iron content of the used oil is due to the presence of metals which arises from the tear and wears in the engine chambers. The engine chamber is made of metals such as aluminum, iron and lead; and the wear of these metals is caused by corrosion and fuel dilution due to bad piston rings [19, 20]. However, treating the used lubricating oil with raw and activated clay samples shows that the treated oil contains 3.01 ppm and 0.30 ppm iron respectively. The reduction in iron content of the used oil when treated with activated clay sample confirmed the ability of clay sample at optimum conditions to remove more iron contaminants, which invariably shows adsorption capacity of the clay sample.
Conclusions
From the analysis of variance, the predicted and experimental values of the responses are almost the same and this signifies that the mathematical models are in agreement with the experimental data. The optimum conditions established in this work are activation temperature of 106.80oC, acid concentration of 3M and activation time of 180 minutes. The recycled oil obtained at the optimum conditions gave a yield of 66.28%, flash point of 105.10oC, cloud point of 14.86oC, specific gravity of 0.881, viscosity of 18.0Cst and iron content of 0.30 ppm. The Brunauer Emmett Teller (BET) surface area of activated clay obtained at optimum conditions was determined to be 437.83m2/g and found to be significantly larger than those of natural porous materials such as clay (10-100 m2g-1). The adsorption capacity of the raw and treated clay was determined to be 1.41 mg/g to 8.64 mg/g respectively and observed to adequately describe the improvement of the adsorption phenomena of the activated clay over raw clay samples.
References
1. Abu-Elella R., Ossman M. E., Farouq R., Abd-Elfatah M., Used Motor Oil Treatment: Turning Waste Oil into Valuable Products, International Journal of Chemical and Biochemical Sciences, 2015, 7(2015), p. 57-67.
2. Udonne J. D., Bakare O. A., Recycling of Used Lubricating Oil Using Three Samples of Acids and Clay as a Method of Treatment, International Archive of Applied Sciences and Technology, 2013, 4(2), p. 8-14.
3. Kojernikova Y. V., Rational ways of preparation of dispensed oil system, 1999, Unpublished Ph.D. Thesis submitted to Russian oil and Gas University, Moscow, pp. 133.
4. Usman M. A., Ekwueme V. I., Alaje T.O., Mohammed A.O., Characterization, Acid Activation and Bleaching Performance of Ibeshe Clay, ISRN Ceramics, 2012, Lagos, Nigeria, doi:10.5402/2012/658508.
5. Emman A. E., Abeer M. S., Re-refining of Used Lube Oil, II- by Solvent/Clay and Acid/Clay-Percolation Processes, ARPN Journal of Science and Technology, 2012, 4, p. 34-41.
6. Udonne J. D., A Comparative Study of Recycling of Used Lubrication Oils using Distillation, Acid and Activated Charcoal with Clay Methods, Journal of Petroleum and Gas Engineering, 2011, 2(2), p. 12-19.
7. Hanrahan G., Lu K., Application of Factorial and Response Surface Methodology in Modern Experimental Design and Optimization, Critical Reviews in Analytical Chemistry 2006, 36, p. 141-151.
8. Yahaya M, Application of Acid Treated Clay in the Recycling of Used Engine Oil. Unpublished Project Submitted to Chemical Engineering Department, 2014, Federal University of Technology Minna.
9. Grim R. E., The Bleaching Clay, 1962, New York; McGraw-Hill, pp. 67-88.
10. Dada A. O., Olalekan A. P., Olatunya A. M., Dada O. Langmuir, Freundlich,Temkin and Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ onto Phosphoric Acid Modified Rice Husk, IOSR Journal of Applied Chemistry 2012, 3(1), p. 38-45.
11. Christidis G. E., Scott P. W., Dumham A. C. Acid activation and bleaching capacity of bentonites from the islands of Milos and Chios, Aegean and Greece, Applied Clay Science, 1997, 12, p. 329-347.
12. Al-Khatib L., Fraige F., Al-Hwaiti M., Al-Khashman O., Adsorption from Aqueous Solution onto Natural and Acid Activated Bentonite, American Journal of Environmental Science, 2012, 8(5), p. 510-522.
13. Bas D., Boyaci I. H., Modeling and Optimization I: Usability of Response Resurface Methodology, Journal of Food Engineering. 2007, 78, p. 836-845.
14. Jabit N. B., The Production and Characterization of Activated Carbon using Local Agricultural Waste through Chemical Activation Process, Unpublished M.Sc. Thesis submitted to the School of Material and Mineral Engineering, 2007, Malaysia. pp. 1-24.
15. Singh J., Uma A., Banerjee S., Gusain D, Sharma Y., Equilibrium Modelling and Thermodynamics of Removal of Orange G from its Aqueous Solution, Journal of Applied Sciences in Environmental Sanitation, 2011, 6(3), p. 317-326.
16. Al-Sultani K. F., Al-Seroury F. A., Characterization the Removal of Phenol from Aqueous Solution in Fluidized Bed Column by Rice Husk Adsorbent Residue, Journal of Recent Sciences, 2012, 1, p. 145-151.
17. Ajemba R. O., Enhancement of Physicochemical Properties of Nteje Clay to increase its Bleaching Performance using Acid Activation, International Journal of Engineering Research and Application, 2012, 2(4), p. 281-288.
18. Hamawand I., Yusaf T., Rafat S., Recycling of Waste Engine Oils Using a New Washing Agent, Energies, 2013, 6, p. 1023-1049.
19. Art J. Lubricating Oil through the Process of Refining Used Motor Oil, 2013. Available at: http://www.ArticleAlloy.com/article.
20. Hamad A., Al-Zubaidy E., Fayed M., Used Lubricating Oil Recycling UsingHydrocarbon Solvents, United Arab Emirate Journal of Environment Management, 2005, 74(2), p. 153-159.