Effect of acid modification on physicochemical characteristics of Kpautagi clay
Matthew A. ONU1,*, Joseph O. OKAFOR1, Abdusalami S. KOVO1 and
Yahaya S. MOHAMMAD2
1Department of Chemical Engineering, F.U.T., P.M.B 65, Minna, Nigeria
2Department of Water Resources and Environmental Engineering, A.B.U., P.M.B 1094, Zaria, Nigeria
E-mails: onu.matthew@yahoo.com; jookai2003@yahoo.com; kovoabdusalami@yahoo.com; yahsaymoh@yahoo.co.uk
*Corresponding author, phone: +2348069081188
Abstract
Chemical activation was carried out on the kaolin clay obtained from Kpautagi Area in Niger State using sulfuric and hydrochloric acids. In order to study the effect of acid activation, the physicochemical characteristics of the Kpautagi clay were determined before and after acid modification and the results obtained were subsequently compared. These characteristics include surface morphology, surface functional groups, surface area and pore volume. Characterization results showed that acid modification of Kpautagi kaolin clay with sulphuric and hydrochloric acids improved the surface area of the clay from 7.598m2/g to 15.5cm2/g and 13.2cm2/g respectively and enhancement in pore volume and average pore diameter were also noted. The changes in the characteristics of the clay after acid modification were further supported by the Scanning Electron Microscope (SEM) micrographs and Fourier Transformed Infrared Spectroscope (FTIR) spectra. Therefore, the outcome of this study shows that sulphuric and hydrochloric acid could be used to improve the characteristics of Kpautagi clay but better performance could be achieved employing sulphuric acid.
Keywords
Surface area; Pore size; Abundant deposit; Micrograph; Spectra; Kaolin; Activation; Materials
Introduction
The most abundant, common and accessible material on the earth crust is clay [1]. Kaolin deposits are ubiquitously spread throughout Nigeria and almost every state in Nigeria has at least one deposit of kaolin [2]. Kaolin consists of a group of hydrous aluminosilicate minerals with chemical formula Al2Si2O5(OH)4 formed by the weathering of feldspathic rocks. The kaolin clay is a layered silicate mineral composed of one tetrahedral sheet which is linked through oxygen atoms to one octahedral sheet of alumina. The major characteristics of kaolinite is its low shrink-swell capacity and a low cation exchange capacity. Kaolin is one type of clay materials which is widely used for a quite number of applications such as paint, paper filling, rubber filler, ceramics wares, paper making, cracking catalyst, cement additive, oil refinery and water treatment. The industrial application of kaolin is strongly connected to its adsorptive properties which depend on surface modification [3, 4]. To the best of our knowledge, there is little or no study on surface (acid) modification of Kpautagi clay. Therefore, the objective of this paper is to study the effect of sulfuric and hydrochloric acids on the characteristics of Kpautagi clay upon modification.
Material and method
Production of activated clay
The clay sample was crushed to obtain smaller particle size using porcelain mortar and pestle. The crushed sample was sieved to obtain particle size of less than75 Ám. 25g of the clay sample was weighed into a 300ml capacity flask and 250ml of 4M sulfuric was added, the resulting suspension was heated under refluxing on a magnetically stirred hot plate at 100oC for 150 minutes. Thereafter, the reaction was quenched with distilled water and the resulting slurry was filtered using Wattman filter paper for separation of the acid from the clay. The obtained residual clay cake was washed several times with distilled water until a pH of 6 to 7 was attained. The obtained product was then dried in the oven for 12 hours at 80oC [3]. The dried samples were then crushed again and sieved to less than 75 Ám particle size. The above procedure was repeated using and 2M hydrochloric acids.
Scanning electron microscopy analysis
The surface morphology of the raw and acid modified clay samples was obtained using Scanning Electron Microscope ( Phenom ProX) working at accelerating voltage of 15 kV, beam size 3.0 and magnification of 2000. The samples were covered with carbon under vacuum before analysis to prevent accumulation of static electric charge on the surface of particles and the microscope images were taken [5, 6].
Fourier transformed infrared spectroscopy analysis
The surface functional groups of the raw and acid modified clay samples were estimated by Fourier Transform Infrared (FTIR) spectroscopy analysis using a spectrometer (Shimadzu 8400s) with smart orbit Attenuated Total Reflectance accessory. The Infrared (IR) spectra of raw and activated clay were recorded from 500-4000 cm-1 at a resolution of 2cm1. The pellet for infrared studies was prepared by mixing a given sample with KBr crystals and pressed into a pellet. The pellet which is homogeneous in appearance was inserted into the IR sample holder for the analysis [5,7].
Surface area and pore size analysis
The surface area and average pore size of the raw and acid modified samples were determined using a gas sorption analyzer (NOVA 4200e Quantachrome and the Quantachrome NovaWin software). The samples were degassed under vacuum at 200oC for 3h prior to measurement. The nitrogen adsorption-desorption data were recorded at liquid nitrogen temperature of 77.35K. The adsorption equilibrium time was set at 60s. The Brunauer, Emmett and Teller (BET) method was used to calculate the surface area, using the data obtained from the N2 adsorption isotherm within the P/Po range of 0.05-0.3, where P is the system pressure and Po is the initial pressure at 1 bar. The micropore volume (Vmic) and the average pore diameter (dp) were derived using DFT method [5, 6].
Results and discussion
Scanning electron microscopy (SEM)
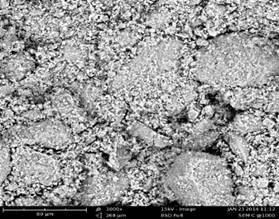
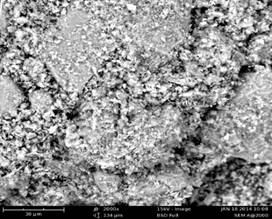
Figures 1 to 3 present the SEM Micrographs of raw, HCl and H2SO4 acid modified clay samples respectively. Comparison of the micrographs revealed improved development of voids and even distribution of pores after acid modification and this could be as a result of leaching of cations during acid activation. Further comparison of the micrographs revealed the presence of large particles that seemed to have been formed due to several flaky particles stacked together in form of agglomerates [8] and the agglomeration is relatively pronounced after acid modification (Figure 2 and 3) as compared to that of raw clay (Figure 1). It could also be observed that HCl modified clay showed less agglomeration when compared with that of the H2SO4 modified clay.
|
|
|
|
Figure 1. Micrograph of raw sample |
Figure 2. Micrograph of HCl modified clay |
|
|
|
Figure 3. Micrograph of H2SO4 modified clay |
Fourier transformed infrared spectroscopy (FTIR)
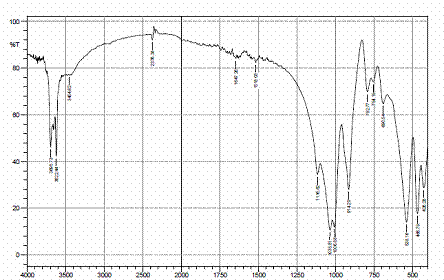
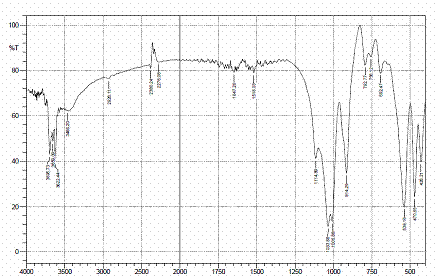
Figures 4, 5 and 6 present the IR spectra for raw, sulphuric and hydrochloric acid modified clay samples. The bands in the region 3620-3695 cm-1 were observed in the three spectra and Pandra et al. [8] reported that these bands correspond to the Al-O-H (structural hydroxyl groups, octahedral). But, a shift in the bands to 3653 cm-1 and 3659 cm-1 were noted after hydrochloric and sulphuric acid modification respectively and this could be as a result of reorganization of the aluminum ions on the surfaces of the clay after acid modification.
|
|
|
Figure 4. FTIR spectra of raw clay |
|
|
Figure 5. FTIR spectra of H2SO4 modified clay |
|
|
|
|
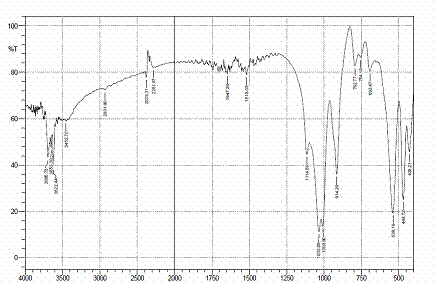
Figure 6. FTIR spectra of HCl modified clay
Surface area and pore size
From the results presented in Table 1, it was observed that the raw clay has surface area of 7.59 m2/g. Thereafter, modification of the clay with hydrochloric and sulphuric acid was observed to improve the surface area of the clay to 13.2 m2/g and 15.2 m2/g respectively.
Table 1. Surface area and pore size of raw and acid modified clay samples
|
Sample |
Surface Area (m2/g) |
Micropore volume (cm3/g) |
Half pore width |
|
HCl |
132.2 |
0.00676 |
1.99 |
|
H2SO4 |
152 |
0.00795 |
1.83 |
|
Raw |
75.9 |
0.00402 |
1.17 |
This is in agreement with our earlier discussion on the SEM micrographs and according to Toor [9] and Kashani et al. [10], this increase could be as result of the removal of impurities, replacement of exchangeable cations with hydrogen or leaching of aluminum ion from the octahedral and tetrahedral sites. Similar findings were also reported by Bhattacharyya and Sengupta [11] and Ajayi et al. [12]. It was also observed that the raw clay possesses pore volume of 0.00402cm3/g but enhancement of the pore volume to 0.00676cm3/g and 0.00795cm3/g was noted after hydrochloric and sulphuric acid modification of the clay. This could be attributed to leaching of aluminum ions resulting in the formation of additional pores. Similarly, the table also shows that enhancement of the average pore diameter from 1.17nm to 1.99nm and 1.87nm was also achieved as a result of the hydrochloric and sulphuric acid modification of the raw clay. This indicates that the raw clay was microporous but acid modification of the raw clay led to a shift making it closer to mesoporosity.
Conclusions
Acid modification of Kpautagi clay showed significant changes in its characteristics which is responsible for its applicability in various areas such as wastewater treatment. Improvement in surface area and enhancement of pore volume and average pore diameter were achieved after sulphuric and hydrochloric acid modification but relatively, sulphuric acid showed better performance. The changes in the characteristics of the clay after acid modification were further supported by the SEM micrographs and FTIR spectra. Therefore, the outcome of this study shows that sulphuric and hydrochloric acid could be used to improve the characteristics of Kpautagi clay but with preference on sulphuric acid.
References
1. Ajayi A. O., Atta A. Y., Aderemi B. O., Adefila S. S., Novel method of metakaolin dealumination-preliminary investigation, Journal of Applied Sciences Research, 2010, 6(10), p. 1539-1546.
2. Ajemba O. R., Modification of the physico-chemical properties of Udi clay minerals to enhance its adsorptive capacity, Pelagia Research Library: Advances in Applied Science Research, 2012, 3(4), p. 2042-2049.
3. Badmus B. S., Olatinsu O. B., Geophysical evaluation and chemical analysis of kaolin clay deposit of Lakiri village, South-Western Nigeria, International Journal of Physical Sciences, 2009, 10, p. 592-606.
4. Bhattacharyya K., gupta S., Adsorption of Fe(III) from water by natural and acid activated clays: studies on equilibrium isotherm, kinetics and thermodynamics of interactions, Adsorption, 2006, 12, p. 185-204.
5. Eze K. A., Nwadiogbu, J. O., Nwankwere, E. T., Effect of acid treatment on the physichochemical properties of kaolin clay, Archives of Applied Science Research, 2012, 4, p. 792-794.
6. John C., Interpretation of Infrared Spectra, a Practical Approach: Encyclopedia of Analytical Chemistry, John Wiley & Sons Limited, 2000.
7. Kashani M. M., Motlagh A. A., Amiri R. Z., Effect of acid activation on structural and bleaching properties of Bentonite, Iranian Journal of Material Science and Engineering, 2011, 8, p. 50-53.
8. Mohammad Y. S., Shaibu-Imodagbe E. M., Igboro S. B., Giwa A., Okuofu C. A., Effect of phosphoric acid modification on characteristics of rice husk activated carbon, Iranica Journal of Energy and Environment, 2015, 6(1), p. 20-25.
9. Olokode O. S., Aiyedun P. O., Mineralogical characteristics of natural kaolins from Abeokuta, South-West Nigeria, The Pacific Journal of Science and Technology, 2011, 12(20), p. 558-565.
10. Panda A. K., Mishra B. G., Mishra D. K., Singh R. K., Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay, Colloids and Surfaces A: Physicochemical andá Engineering Aspects, 2010, 363, p. 98-104.
11. Toor M. K., Enhancing adsorption capacity of Bentonite for dye removal: Physicochemical modification and characterization, M.Sc Thesis, The University of Adelaide, Australia, 2010.
12. Vimonses V., Development of multifunctional nanomaterials and adsorption-photocatalysis hybrid system for wastewater reclamation, Journal of Hazardous Materials, 2011, 44, p. 5385-5397.