Characterization and application of Mucuna urens seed husk as novel biosorbent for sequestration of some divalent metal ions from solution
Linda Obiageli ELUKE1, Kovo Godfrey AKPOMIE2,*, Helen Ogechi CHUKWUEMEKA-OKORIE1,3, Vincent Ishmael AJIWE1
1Department of Pure and Industrial Chemistry, Nnamdi Azikiwe University, P.M.B 5025, Awka, Anambra State, Nigeria
2Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Enugu State, Nigeria
3Department of Chemistry, Michael Okpara University of Agriculture, Umudike, P.M.B 7267, Umuahia, Abia State, Nigeria
E-mail(*): kovo.akpomie@unn.edu.ng
*Corresponding Author, phone: +2348037617494
Abstract
The utilization of Horse-Eye Bean (Mucuna urens) Seed Husk (HEBSH) as low cost biosorbent for the abstraction of Co (II), Pb (II) and Zn (II) ions from water was performed. The biosorbent was characterized by the Fourier Transform Infrared (FTIR) spectroscopy, X-ray diffraction (XRD) and Scanning Electron Microscopy (SEM). The influence of pH, initial metal ion concentration, biosorbent dosage and contact time on the biosorption was studied. Four isotherm and four kinetic models were used to determine the mechanism of biosorption. FTIR showed the presence of some functional groups such as OH, C=O, C=C, C-O, C-Cl on the surface of HEBSH, while XRD and SEM showed amorphous and non-porous surface structure respectively. Optimum biosorption of the metal ions was obtained at pH 6.0, biosorbent dosage 0.1 g, metal concentration 400 mg/L and contact time of 30, 40, and 60 min for Co (II), Zn (II) and Pb (II) respectively. The Freundlich gave the best fit for the three metal ions (R2 > 0.95) than the Langmuir, Tempkin and Dubinin-Radushkevich models indicating a multilayer biosorption on a heterogenous surface of HEBSH. Kinetic analysis showed the pseudo first order model presented good fits for Zn (II) and Pb (II) while the pseudo second order model for Co (II). The initial sorption rate h (mg/g min) was 2.35 × 10-3, 2.1 × 10-4 and 6.3 × 10-7 for Co (II), Zn (II) and Pb (II) ions respectively. Liquid film diffusion was found to be the diffusion mechanism of biosorption rather than the intraparticle diffusion. The experimental investigation showed that HEBSH is effective as a low cost alternative biosorbent for the sequestration of heavy metal ions from aqueous stream.
Keywords
Biosorption; Cobalt; Horse eye bean; Kinetics; Lead; Seed husk; Zinc
Introduction
Environment pollution of toxic heavy metals from industrial effluents is of serious concern to many industrialized Nations. This is because these metals are very toxic at certain concentrations, non-biodegradable, persist in the environment and bio-accumulate in the food chain [1]. As a result of industrial development, the problem posed by theses toxic metals to the environment has been on the increase. Heavy metals from industrial effluents get to receiving water bodies affecting aquatic lives which in turn affect humans feeding on such due to bioaccumulation in the human body. This results in various kinds of illnesses such as kidney and liver damages, skin diseases, cancer and even death [2]. Lead for instance in high concentrations can damage the brain and nervous system. Cobalt exposure may result in weight loss, dermatitis, bleeding, vomiting, sterility, hair loss, coma and respiratory hypersensitivity. Long term ingestion of zinc contaminated drinking water causes skin, lungs and kidney cancer, gastrointestinal disease, bone marrow disorder and cardiovascular diseases. Therefore such heavy metals must be removed from effluents before discharge into receiving water bodies [3].
A number of techniques have been utilized in the removal of heavy metals from industrial effluents such as solvent extraction, electrodialysis, membrane filtration, evaporation, coagulation, reverse osmosis, ion exchange and precipitation [2]. These techniques have disadvantages in terms of low metal removal efficiency, require skilled personnel’s, high capital and operational cost and the disposal of residual sludge of metal ions [4, 5]. However, the adsorption technique has been found to be the most effective and activated carbon the most efficient adsorbent with high adsorption capacity [6]. The limitation of the use of activated carbon is that it is expensive which is not favorable to most developing nations and tend to increase the cost of industrial production. Therefore, a lot of extensive research has been carried out by many researchers on the use of alternative low cost materials as adsorbents for heavy metals [2, 7-10].
Biosorption using agricultural waste or biomass material such as amaranthus hydidus stalk waste [1], ficus glomerata [8], Banana peels [9], orange peel [10], citrus peels [11] jackfruit peel [12] cicer arientinum husk [13] have been found utilized effectively for the removal of heavy metals from solution. However, despite the availability of horse-eye bean in most developing and other developed nations, a thorough literature search revealed a complete lack of information on the use of its seed husk as low cost alternative adsorbent for heavy metals. In this regard, this study therefore reports for the first time the potentials of horse-eye bean seed husk as low cost adsorbent for the sequestration of Zn (II), Pb (II) and Co (II) ions from aqueous solution. The biosorbent was used without chemical treatment or modification in order to maintain a low process cost. The kinetic and equilibrium mechanistic models of biosorption were also investigated.
Material and method
Biosorbent preparation and characterization
The Horse eye bean was purchased from Ogbete market, Enugu, Nigeria. They were then sundried for 7 days after which the husk was removed manually. The removed husk were sundried further for another 7 days after which it was pulverized and passed through a mesh sieve of size 100 µm to obtain the Horse-Eye Bean Seed Husk (HEBSH) biosorbent. The HEBSH was then stored in a pretreated plastic container until use for the biosorption process.
The HEBSH was then characterized by the use of the Fourier Transform Infrared (FTIR) Spectrophotometer (Shimadzu FTIR 8400s), X-ray diffractometer (Randicon MD 10 model) and the Scanning Electron Microscope (SEM) (Hitachi S4800 model).
Adsorbate preparation and biosorption study
All the reagents used in this study were of analytical grade, 1000 mg/L solution of cobalt (II), lead (II) and zinc (II) ions were prepared by dissolving appropriate amounts of Co(NO3)2, Pb(NO3)2 and Zn(NO3)2 in de-ionized water. Lower concentrations (200 – 800 mg/L) were prepared from the stock by serial dilution. The pH of each solution was adjusted to values from 2.0 – 8.0 by the addition of 0.1M NaOH and 0.1M HNO3 drop by drop when required. Batch biosorption was performed using HEBSH and metal solutions at pH (2.0 – 8.0), initial metal ion concentration (200 – 1000mg/L), adsorbent dosage (0.1 – 0.5 g) and contact time (5 – 120 min). This was performed by contacting 0.1 g of HEBSH with 20 mL of a given solution in 100 mL pretreated plastic containers at room temperature of 270C. To investigate the effect of a parameter, that parameter was varied while other parameters were kept constant at the optimum biosorption values of pH 6.0 and 400 mg/L metals solution. At the end of a given contact time of biosorption the solution was filtered and the filtrate was analyzed for residual metal ion concentration using the Atomic Absorption Spectrophotometer (AAS) (Buck Scientific Model, 210VGP).Each experiment was performed in duplicate twice and the mean value calculated. The percentage biosorption and biosorption capacity of HEBSH for metal ions were calculated as described previously [14].
Biosorption isotherm algorithms
Biosorption isotherms are important in description of how the affinity of biosorbents for metal ions and are useful in the design of adsorption systems. The Langmuir, Freundlich, Temkin and Dubinin-Radushkevich (D-R) isotherm models were applied to the biosorption.
The Langmuir isotherm describes a monolayer adsorption on a homogenous biosorbent surface and is given in its linear form as [15]:
|
Ce/qe = 1/qLKL + Ce/qL |
(1) |
where Ce (mg/L) is the equilibrium concentration of metal ions, qe (mg/g) is the adsorption capacity at equilibrium, qL (mg/g) is the Langmuir monolayer adsorption capacity and KL (L/mg) is a constant related to the energy of adsorption. This model was applied by a linear plot of Ce/qe against Ce. A separation factor (RL) of the Langmuir model was utilized to describe the biosorption and expressed as [3]:
|
RL = 1/[1 + KLCo] |
(2) |
where Co (mg/L) is the initial concentration of metal ions in solution. The value of RL indicated the type of biosorption to be irreversible (RL = 0), favorable (0< RL< 1), linear (RL = 1) or unfavorable (RL > 1).
The Freundlich isotherm describes a multilayer adsorption on a heterogeneous biosorbent surface and the linear form of the equation is given as [16]:
|
logqe = logKF + [1/n]logCe |
(3) |
where KF (L/g) and n are the Freundlich constants related to the biosorption capacity and intensity, respectively. The Freundlich model was applied by a linear plot of logqe versus logCe.
The Temkin model assumes that the free energy of biosorption is a function of the surface coverage and the linear form of the equation is given as [3]:
|
qe = BlnA + BlnCe |
(4) |
where A (L/mg) is the equilibrium binding constant and B (mg/g) corresponds to the heat of biosorption. The Tempkin model was applied by plotting qe against Ce values.
The Dubinin-Radushkevich (D-R) model does not assume a homogenous biosorbent surface and was applied in its linear form as [7]:
|
lnqe = lnqm - βε2 |
(5) |
where β (mol2/J2) is a D-R constant related to the mean free biosorption energy, qm (mg/g) is the theoretical saturation capacity, ε = RTln(1 + 1/Ce) is the Polanyi potential, R (8.314 J/mol K) is the ideal gas constant and T (K) is the absolute temperature. A linear plot of lnqe versus ε2 was used to evaluate the applicability of the D-R model to the biosorption process.
Kinetic algorithms
Kinetic analysis was carried out to determine the rate of biosorption of metal ions on HEBSH by the application of the Pseudo-First Order (PFO), Pseudo-Second Order (PSO), Intraparticle Diffusion (ID) and Liquid Film Diffusion (LFD) models.
The PFO model assumes that the rate of biosorption site occupation is proportional to the number of unoccupied sites and is expressed linearly as [17]:
|
log(qe – qt) = logqe – (KI/2.303)t |
(6) |
where qt (mg/g) corresponds to adsorption capacity at time t (min) and KI (min-1) is the PFO rate constant. The PFO model was applied by a linear plot of log(qe – qt) versus t.
The PSO model assumes that chemisorption is the rate controlling mechanism of biosorption and is expressed in its linear form as [17]:
|
t/qt = 1/h + t/qe |
(7) |
where h = K2qe2, represents the initial biosorption rate (mg/gmin), K2 (g/mg min) is the PSO rate constant. The linear plot of t/qt against t was used to evaluate the applicability of PSO model to the biosorption process.
The ID model is applicable when the transport of metal ions from the biosorbent surface to the intra-particle active sites (pores) is the major mechanism of biosorption. The linear form of ID model equation is expressed as [18]:
|
qt = Kdt1/2 + C |
(8) |
where Kd (mg/g min1/2) is the ID rate constant and C corresponds to the boundary layer effect. This model was applied by a linear plot of qt versus t1/2 and if it passes through the origin then ID is the sole rate determining step. Otherwise the intercept indicates the existence of a degree of surface phenomenon.
The LFD mechanism applies to the biosorption process when the transport of metal ions from the bulk to the biosorbent surface plays the most significant role and is expressed as [18]:
|
ln(1 – F) = Kfdt + P |
(9) |
where Kfd (mg/gmin) is the LFD rate constant, F (qt/qe) is the fractional equilibrium attainment. A linear plot of –ln(1 – F) versus t indicate the presence of LFD, and if P ≠ 0 then LFD is not the sole rate controlling mechanism of biosorption.
Results and discussion
FTIR, XRD and SEM analysis of HEBSH
The FTIR spectrum of HEBSH is shown in Fig. 1. The FTIR provides useful information on the surface functional groups of the adsorbent responsible for biosorption of metal ions. The broad band observed at 3419.9 cm-1 indicated the presence of OH sites on the surface of HEBSH. Absorption bands at 2374.5 cm-1 corresponded to the C-H stretching vibration of aliphatics while the C=O group was indicated by the band at 1643.4 cm-1[19]. Bands at 1464.0 cm-1 represented the C=C group of alkenes or the symmetric carbonyl stretching of carboxylic groups, while the bands at 1267.3 cm-1 and 1134.2 cm-1 could be attributed to the C-O stretching vibrations of carboxylic acid and alcohols [20]. The band at 667.39 cm-1 was attributed to the C-Cl stretching vibration or the C-H out of plane bands of alkanes [19]. Thus the FTIR revealed the presence of surface functional groups on HEBSH for efficient sequestration of Pb (II), Zn (II) and Co (II) from solution.
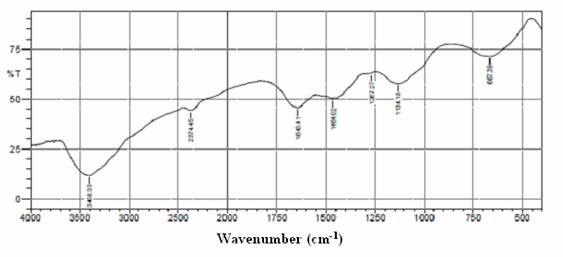
Figure 1. Fourier Transform Infrared Spectra of HEBSH
The XRD spectrum of HEBSH is shown in Fig. 2, the spectrum showed weak and broad peaks which indicated the amorphous nature of the biosorbent.
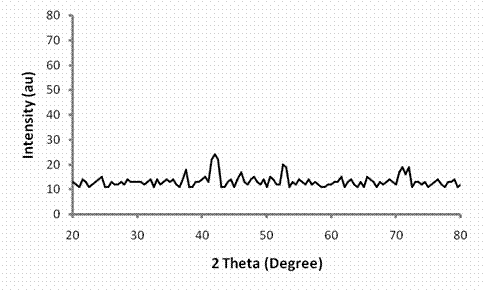
Figure 2. X-ray diffraction spectrum of horse-eye bean seed husk
The amorphous nature suggests that metal ions can easily penetrate the surface of HEBSH for their sorption which is desirable [21]. The SEM morphology of HEBSH is shown in Fig. 3, and revealed an irregular surface, with particle aggregation of various shapes and sizes. It is observed that the surface of the adsorbent is not porous; hence the likely mechanism of biosorption of the metal ion on HEBSH should be film diffusion rather than the intraparticle diffusion mechanism.
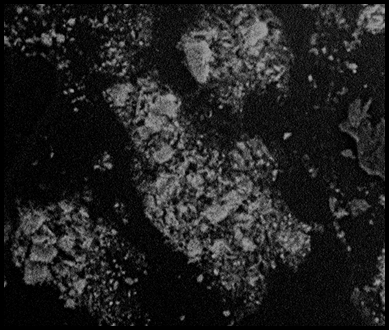
Figure 3. SEM morphology of Horse-eye bean seed husk
pH effect of metal ions biosorption on HEBSH
The initial solution pH is important in biosorption as the protonation of functional groups on the adsorbent surface and chemistry of metal ions are strongly affected by the pH. The influence of pH on the biosorption of the three metal ions on HEBSH is shown in Fig.4. The result showed an increase in biosorption of the metal ions with pH increase. The biosorbent recorded an increase from 5 to 63.75%, 3.75 to 63% and 4 to 55.75% with pH increase from 2.0 to 8.0 in the biosorption of Co (II), Pb (II) and Zn (II) respectively. The increase in biosorption with pH is attributed to the decrease in H+ ions concentration in solution [22]. At lower pH values the solution is acidic and has high concentration of H+ ions in solution which competes with the positively charged metal ions for the active sites on the biosorbent leading to less removal of metal ions. However as the pH increases the solution becomes less acidic and the number of H+ ions in solution decreases making more sites available for metal biosorption due to decrease in competition. Similar results have been reported by other scientists [1, 7, 15].
It was also observed that Pb (II) was biosorbed more than Co (II) and Zn (II) at pH 3.0 to 5.0 and attained optimum removal at 5.0. Optimum biosorption pH for Co (II) and Zn (II) ions was 6.0. The trend recorded at optimum values was Co > Pb > Zn ions. The pH of 6.0 was utilized and maintained in subsequent biosorption experiment as optimum percentage removal was recorded and metal ions precipitation associated with higher pH values were avoided [23]. Similar result was reported [3].
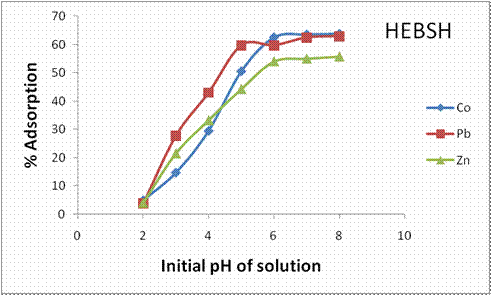
Figure 4. Influence of initial metal ion concentration on biosorption
The influence of initial concentration of metal ions on the biosorption capacity of HEBSH for Pb (II), Zn (II) and Co (II) ions is shown in Fig.5. An increase in the biosorption capacity of HEBSH with increase in the initial concentration of the three metal ions was obtained. The biosorption capacity increased from 32 to 76.8 mg/g, 30.4 to 73.8 mg/g and 28.2 to 68.6 mg/g with increase in metal concentration from 200 – 1000mg/L for Co (II), Pb (II) and Zn (II) ions respectively. The increase in biosorption capacity with increase in metal concentration is due to increasing concentration gradient which acts as a driving force to overcome the resistance to mass transfer resulting in increase in collision between the adsorbate and adsorbent [24].
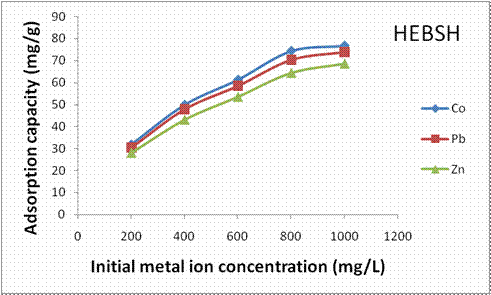
Figure 5. Effect of initial metal ion concentration on the adsorption capacity of heavy metals on HEBSH
On the other hand, the effect of initial metal ion concentration on the percentage biosorption of metal ions by HEBSH is shown in Fig. 6. A decrease in percentage biosorption of the three metal ions on the biosorbent with increase in metal concentration was observed. With increase in metal concentration from 200 to 1000 mg/L the percentage biosorption of Co (II), Pb (II) and Zn (II) ions on GCSH decreased from 80 to 38.4%, 76 to 36.9% and 70.5 to 34.3% on HEBSH respectively. The decrease is attributed to decrease in the number of active sites available at higher metal ion concentration due to saturation of the active sites as concentration increases [25]. The trend of metal biosorption on HEBSH was Co > Pb > Zn ions. The electronegativity and ionic radii of the metal ions were accountable for the higher biosorption of a metal ion relative to another recorded in this study. Metals with higher electronegativity are more attracted to the negatively charged functional groups of the biosorbent surface than metals with lower electronegativity [18]. In this regard, considering the electronegativity of the three metal ions, Co (II) (1.88) > Pb (II) (1.87) > Zn (II) (1.65), Co (II) and Pb (II) tend to be biosorbed more than Zn (II). However, the higher biosorption of Co (II) than Pb (II) ions is due to the smaller ionic radius of Co (II) (0.74Å) than Pb (II) (1.20Å) which allowed for an easy diffusion of Co (II) ions to get to the surface of HEBSH than the larger Pb (II) ions [18]. Similar results have been reported [7, 21].
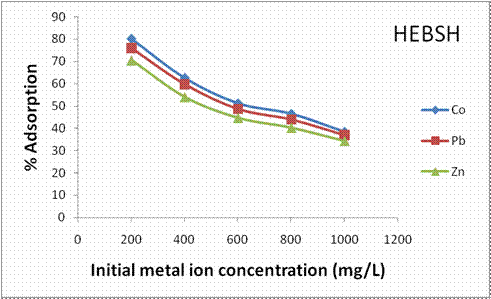
Figure 6. Effect of initial metal ion concentration on the percentage adsorption of heavy metals by HEBSH
Influence of biosorbent dosage on biosorption
The influence of biosorbent dosage on the biosorption capacity of HEBSH for Co (II), Pb (II) and Zn (II) ions is shown in Fig. 7. An observed decrease in the biosorption capacity of the biosorbent for the three metal ions with increase in biosorbent dosage was recorded. With increase in biosorbent dosage from 0.1 to 0.5 g a decrease in the biosorption capacity from 50 to 12.93 mg/g, 47.8 to 12.56 mg/g and 43.2 to 12.2 mg/g was obtained for the respective metal ions. The decrease is attributed to increase in the number of active sites available for biosorption with increase in dosage resulting in unsaturation of the active sites. It is also due to an increase in diffusion path length resulting from overlapping or aggregation of biosorptive active sites [26].
Furthermore, the effect of biosorbent dosage on the percentage biosorption of metal ions by HEBSH is shown in Fig.8. An opposite trend was observed where an increase in percentage biosorption of the three metal ions was recorded. With increase in adsorbent dose from 0.1 to 0.5 g the percentage biosorption of Co (II), Pb (II) and Zn (II) increased from 62.5 to 80.75, 59.75 to 78.5% and 54 to 76.25% respectively. The increase is attributed to increase in the number of active biosorption sites and surface area of the biosorbent as well as decrease in the electrostatic potential near the surface [14]. Similar results have been reported [3, 27]. The trend of biosorption was in the order Co > Pb > Zn, which corroborated that obtained from the effect of metal ion concentration. The biosorbent dosage of 0.1 g was utilized in all the biosorption experiments in this study for maximum utilization of the actives sites on HEBSH.
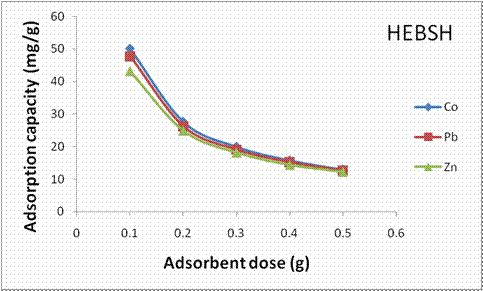
Figure 7. Effect of adsorbent dose on the adsorption capacity of HEBSH for heavy metals
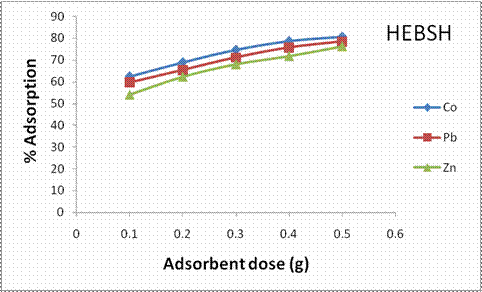
Figure 8. Effect of adsorbent dose on the percentage adsorption of heavy metals by HEBSH
Influence of contact time on biosorption
The time it takes metal ions and biosorbent to reach equilibrium is of considerable importance as it provides information on the process mechanism. Therefore it is necessary to determine the effect of contact time for any biosorption process. The influence of contact time on the biosorption of Co (II), Pb (II) and Zn (II) ions on HEBSH is shown in Fig. 9. It was observed that the rate of metals biosorption was initially rapid and then diminished gradually until an equilibrium time beyond which there was no significant increase in the rate of removal. An equilibrium contact time of 30, 60 and 40 min was observed for Co, Pb and Zn ions respectively. It nature of the biosorbent and its available actives sites have been reported to be responsible for different equilibrium times recorded by metal ions [3]. However, the metals ions all recorded different contact times on the same biosorbent, suggesting that the difference in the rate of biosorption is as a result of differences in the properties of metal ions. The ionic radius of metal ions has been reported to play the most significant role as metals with smaller ionic radii tend to diffuse faster having a faster rate of biosorption and vice versa [28]. Considering the ionic radius of the metal ions; Co (0.74Å), Zn (0.74Å) and Pb (1.20Å) and the equilibrium biosorption times Co (30 min), Zn (40 min) and Pb (60 min). Co and Zn had faster rate of biosorption than Pb due to their smaller ionic radius, and the faster rate obtained for Co than Zn was due to the higher electronegativity of the former as discussed previously. The increase in biosorption of metal ions with contact time is due to the rapid filling of the abundant active sites on the surface of HEBSH which becomes used up with time (saturated) thus attaining equilibrium [14]. The fast biosorption kinetics suggested the applicability of the biosorption process. A contact time of 120 min was utilized in this experiment to enable equilibrium biosorption of all the metal ions. Similar results have been reported by some workers [20, 23].
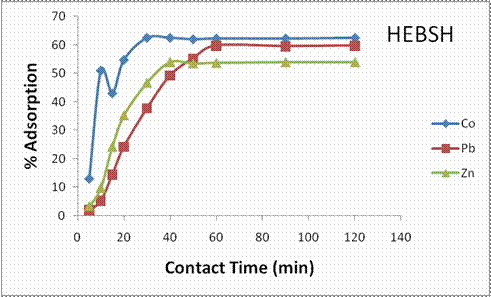
Figure 9. Effect of contact time on the percentage adsorption of heavy metals on HEBSH
Biosorption isotherm
In this research the Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherm models analysis were applied in the biosorption of Co (II), Pb (II) and Zn (II) ions on the adsorbents. The most suitable isotherm is determined by the linear regression coefficient (R2). The closer the R2 value of the model plots to one the best the fit of the model [21]. The Langmuir model plots for the biosorption of the metal ions on HEBSH are shown in Fig.10 and the isotherm parameters are presented in Table 1.
Table 1. Equilibrium isotherm model parameters for the biosorption process
|
Isotherm/Adsorbent |
Co (II) |
Pb (II) |
Zn (II) |
|
Langmuir |
|||
|
qL (mg/g) |
90.91 |
90.91 |
83.33 |
|
KL (L/mg) |
0.010 |
0.008 |
0.007 |
|
R2 |
0.988 |
0.989 |
0.984 |
|
Freundlich |
|||
|
KF |
9.44 |
7.83 |
5.90 |
|
n |
3.02 |
2.84 |
2.61 |
|
R2 |
0.991 |
0.994 |
0.991 |
|
Tempkin |
|||
|
B (mg/g) |
16.98 |
17.22 |
17.42 |
|
A (L/g) |
0.149 |
0.112 |
0.077 |
|
R2 |
0.976 |
0.983 |
0.970 |
|
Dubinin-Radushkevich |
|||
|
qm (mg/g) |
66.22 |
63.49 |
59.09 |
|
B (mol2/J2) |
0 |
0 |
0 |
|
R2 |
0.818 |
0.831 |
0.810 |
It was observed that the values of the linear regression coefficient (R2) obtained were high and gave good fits to the experimental data (R2 values < 0.9). However, the R2 values were lower than those obtained for the Freundlich model. This suggested that the biosorption process is likely not a monolayer one and that the surface of HEBSH is not homogenous in nature. Also, the values of RL obtained for the biosorption of Co (II), Pb (II) and Zn (II) ions on HEBSH were in the range of 0.059 to 0.417. This corresponded to a favorable biosorption process indicating that the HEBSH has great potentials as an efficient biosorbent for the removal of toxic metal ions from contaminated solution.
The Freundlich model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions on HEBSH are shown in Fig.10 and the model parameters are presented in Table 1. It was observed that the Freundlich model provided the best fit than among the four isotherm models with R2 values all greater than 0.990. This clearly indicated that the biosorption of the three metals ions is a multilayer one on a heterogenous surface of HEBSH. This is supported by the fact that the FTIR results showed the existence of more than one kind of functional sites on the surface. Furthermore, the values of n between 1 and 10 indicates a favorable biosorption process between the biosorbent and metal ions [27]. The values of n obtained for the biosorption of Co (II), Pb (II) and Zn (II) ions on HEBSH, are within the range of 2.61 – 3.02 which also indicated as in the case of the Langmuir RL values a favorable biosorption of metal ions HEBSH.
The Tempkin isotherm model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions HEBSH are shown in Fig. 11 and the isotherm parameters are given in Table 1. The R2 obtained from the Temkin model were high and presented good fits (R2 > 0.9). However they were lower than those of the Langmuir and Freundlich model which indicated that the Tempkin’s model was not appropriate in the description of the biosorption of the studied metal ions on HEBSH.
The D-R isotherm model plots obtained for the biosorption of Co (II), Pb (II) and Zn (II) ions HEBSH are shown in Fig. 11 and the isotherm parameters are given in Table 1. The R2 values (<0.9) indicated that this model did not provide a good fit to the biosorption process. This model presented the least fit and is therefore not suitable in the description of the mechanism of biosorption. Signifiantly in this isotherm analysis is the fact that HEBSH was found to be suitable for the biosorption of metal ions from solution as deduced from the Langmuir RL and Freundlich n values. Similar results have been reported by other researchers [1, 3, 16, 24, 26].
Biosorption kinetic analysis
Information on the kinetics of metal uptake is required to select the optimum condition for full-scale batch metal removal processes. Predicting the rate of biosorption is among the most important factors in the design of an adsorption system. This is because the system kinetics determines adsorbate residence time and the reactor dimensions [18]. The PFO, PSO, ID, LPD rate equation were applied in the biosorption of Co (II), Pb (II) and Zn (II) ions from solution. The PFO plots for the biosorption of the metal ions are shown in Fig.12 while the model parameters obtained are presented in Table 2.
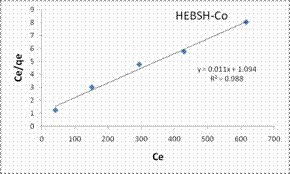
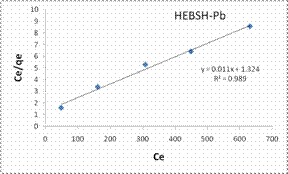
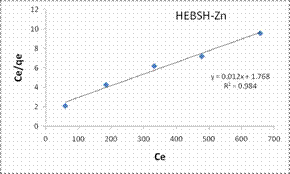
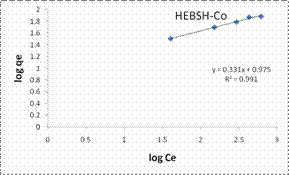
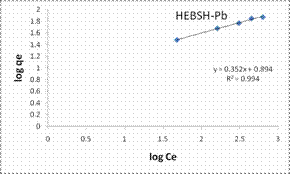
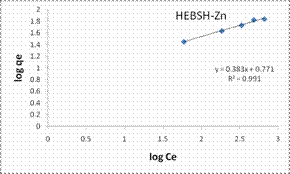
Figure 10. Langmuir and Freundlich isotherm model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions on GCSH
The good fit of the PFO model to the data indicated the mechanism of biosorption to be physisorption and might have proceeded mainly through liquid film diffusion [21]. This model was compared to that obtained from the PSO. The PSO model plots for the biosorption of the three metal ions are shown in Fig.12 and the model constant parameters are given in Table 2. This model have been found to give the best fit in most biosorption studies [1, 6, 13, 15], but a different result was obtained here. However this model presented a very good fit for the biosorption of Co (II) than the PFO model but the biosorption of Pb (II) and Zn (II) had low R2 values. Furthermore the experimental qe values were close to the qe calculated for Co (II), indicating a chemisorptions mechanism for its biosorption on HEBSH.
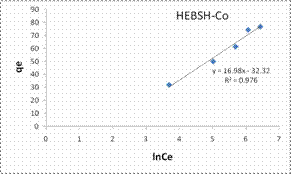
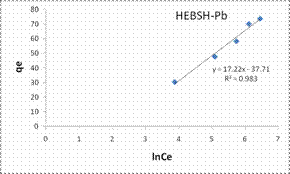
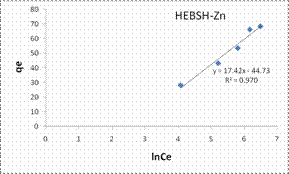
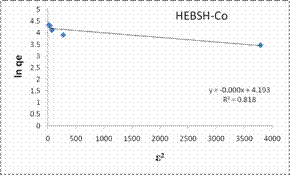
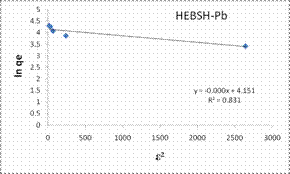
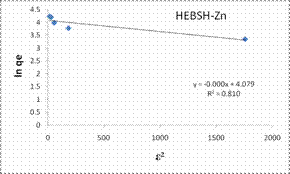
Figure 11. Tempkin and Dubinin-Radushkevich isotherm model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions on GCSH
However, those of Pb (II) and Zn (II) ions were better described by the PFO model suggesting a physical adsorption process [7].
Also, the initial sorption rate (h) showed a faster biosorption of Co (II) followed by Zn (II) and a much slower rate for Pb (II) ions. This result supported that obtained from the effect of contact time as equilibrium times of 30, 40 and 60 min were obtained for the metal ions respectively.
To explore the diffusion mechanism of the biosorption process the ID and LFD models were analyzed. The ID model plots for the biosorption of the metal ions are shown in Fig.13 and the model constant parameters are presented in Table 2. The R2 obtained were low (R2 < 0.9), which indicated a poor fit of this model suggesting that ID is not the rate controlling mechanism of biosorption [21]. The plots did not pass through the origin due to the occurrence of the intercept C, indicating that ID is not the sole rate controlling step even if present [27]. The deviation from the origin might be due to the differences in the rate of mass transfer in the initial and final stages of the process [18]. Furthermore, the lower R2 values and the occurrence of the intercepts suggested that LFD is the rate controlling mechanism of biosorption. This was verified after the analysis of the LFD model. The LFD model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions are shown in Fig.13 while the model parameters are given in Table 2. As observed the R2 values were high (> 0.9) and much better than the ID model, except the biosorption of Co (II) on HEBSH which was 0.656 but was still higher than that obtained from the ID model of 0.492. This confirmed that film diffusion played a dominant and relevant role as the rate controlling mechanism in the biosorption of metal ion on HEBSH. Also, the occurrence of the intercept suggested that it was not the sole rate determining step but some degree of ID mechanism might have been involved. Similar results have been reported [3, 18]. The greater contribution of LFD and physisorption is desirable as easy desorption of metal ions from the biosorbent can be achieved when reuse of the biosorbent is required [7].
Table 2. Kinetic model parameters for the biosorption process
|
Kinetic/Adsorbent |
Co (II) |
Pb (II) |
Zn (II) |
|
qeexp (mg/g) |
50 |
47.8 |
43.2 |
|
Pseudo-first-order |
|||
|
qecal (mg/g) |
48.19 |
78.52 |
71.61 |
|
KI (min-I) |
0.099 |
0.055 |
0.081 |
|
R2 |
0.906 |
0.966 |
0.975 |
|
Pseudo-second-order |
|||
|
qecal (mg/g) |
55.56 |
1000 |
76.92 |
|
h(mg/g min) |
7.246 |
0.631 |
1.238 |
|
K2(g/mg min) |
2.35 × 10-3 |
6.3 × 10-7 |
2.1 × 10-4 |
|
R2 |
0.978 |
0.007 |
0.513 |
|
Intraparticle diffusion |
|||
|
Kd (mg/g min1/2) |
3.159 |
6.184 |
4.819 |
|
C |
23.65 |
-8.22 |
1.77 |
|
R2 |
0.492 |
0.852 |
0.725 |
|
Film diffusion |
|||
|
Kfd |
0.100 |
0.057 |
0.080 |
|
P |
0.035 |
0.496 |
0.507 |
|
R2 |
0.656 |
0.966 |
0.975 |
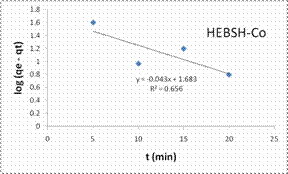
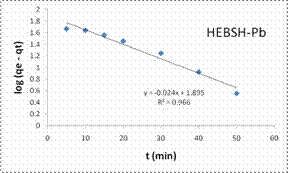
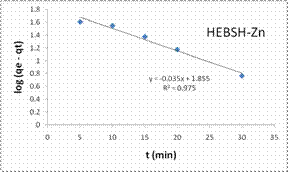
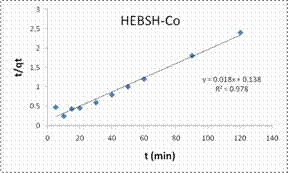
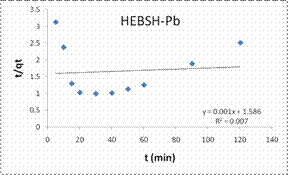
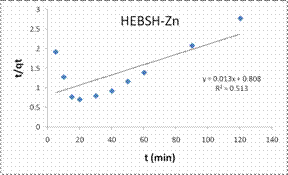
Figure 12. Pseudo first order and pseudo second order plots for the biosorption of Co (II), Pb (II) and Zn (II) ions from solution on HEBSH
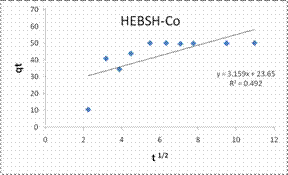
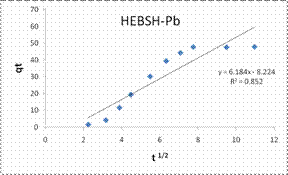
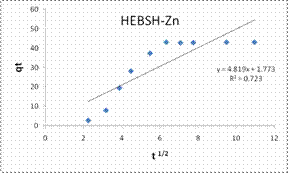
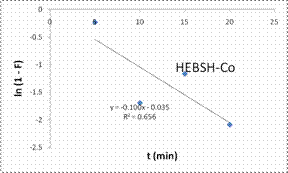
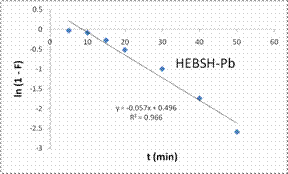
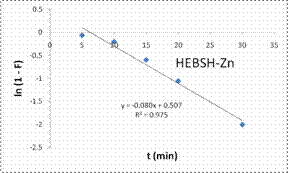
Figure 13. Intraparticle duffusion an liquid film diffusion model plots for the biosorption of Co (II), Pb (II) and Zn (II) ions from solution on HEBSH
Conclusion
It was discovered from the experimental result and analysis that horse-eyed bean seed husk had several surface functional groups and amorphous nature as revealed from the Fourier transform infrared and X-ray diffraction respectively. The Scanning electron microscopy revealed a non-porous surface nature of the biosorbent indicating a film diffusion mechanism which was confirmed by the good fit of the liquid film diffusion model to the biosorption. The Freundlich model was found to present the best fit among the four isotherm models tested which indicated a multilayer biosorption on a heterogeneous surface of horse-eye bean seed husk. Importantly, the biosorbent was found to be suitable and effective in the removal of the three metal ions from solution as deduced from the Langmuir and Freundlich models. This suggested that horse-eye bean seed husk can be utilized as an efficient low cost alternative adsorbent to activated carbon for the sequestration of unwanted metal ions from water.
References
1. Egila J. N., Dauda B. E. N., Jimoh T., Biosorptive removal of cobalt (II) ions from aqueous solution by amaranthus hydidus L. stalk waste, African Journal of Biotechnology, 2010, 9, p. 8192-8198.
2. Sorgho B., Mahamane A. A., Guel B., Zerbo L., Gomina M., Blanchart P., Removal of Cd,Cu and Pb with a Bukina Faso clay, Scientific Study and Research, 2016, 17, p. 365-379.
3. Akpomie K. G., Dawodu F. A., Treatment of an automobile effluent from heavy metal contamination by an eco friendly montmorillonite, Journal of Advanced Research, 2015, 6, p. 1003-1013.
4. Kurniawan T. K., Chan G. Y. S., Lo W., Babel S., Comparison of low cost adsorbents for treating wastewaters laden with heavy metals, Science of Total Environment, 2006, 366, p. 409-426.
5. Abuh M. A., Akpomie K. G., Nwagbara N. K., Abia-Bassey N., Ape D. I., Ayabie B. U., Kinetic rate equations application on the removal of copper (II) and zinc (II) by unmodified lignocellulosic fibrous layer of palm tree trunk: single component system studies, International Journal of Basic and Applied Sciences, 2013, 1, p. 800-809.
6. Zenasni M. A., Benfarhi S., Merlin A., Molina S., Meroufel B., Adsorption of Cu (II) on maghnite from aqueous solution: effect of pH, initial concentration, interaction time and temperature, Natural Science, 2012, 4, p. 856-868.
7. Dawodu F. A., Akpomie K. G., Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite clay, Journal of Materials Research and Technology, 2014, 3, p.129-141.
8. Rao R. A. K., Rehman F., Adsorption studies on fruits of gular (ficus glomerata): removal of Cr(VI) from synthetic wastewater, Journal of Hazardous Material, 2010, 181, p. 405-412.
9. Anwar J., Shafique U., Zaman W., Salman M., Dar A., Anwar S., Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana, Bioresource Technology, 2010, 101, p. 1752-1755.
10. Ningchun F., Guo X., Liang S., Zhu Y., Liu J., Biosorption of heavy metals from aqueous solution by chemically modified orange peel, Journal of Hazardous Material, 2011, 185, p. 49-54.
11. Schiewer S., Patil S. B., Modeling the effect of pH on biosorption of heavy metals by citrus peels, Journal of Hazardous Material, 2008, 157, p. 8-17.
12. Inbaraj S., Sulochana N., Carbonised jackfruit peel as an adsorbent for the removal of Cd (II) from aqueous solution, Bioresource Technology, 2004, 94, p. 49-52.
13. Ahalya N., Kanamadi R. D., Ramachandra T. V., Biosorption of iron (III) from aqueous solution using the husk of cicer arientinum, Indian Journal of Chemical Technology, 2006, 13, p. 122-127.
14. Etukudoh A. B., Akpomie K. G., Obi N. D., Chimezie P. E., Agbo A. E., The potential of a natural clay mineral (nsu clay) for the adsorption of lead (II) ions from aqueous stream, Der Pharma Chemica, 2016, 8, p. 9-15.
15. Bhatnagar A., Minocha A. K., Sillanpaa M., Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent, Biochemical Engineering Journal, 2010, 48, p. 181-186.
16. Reddy N. A., Lakshmipathy R., Sarada N. C., Application of Citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution, Alexandria Engineering Journal, 2014, 53, p. 969-975.
17. Lasheen M. R., Ammar N. S., Ibrahim H. S., Adsorption/desorption of Cd (II), Cu (II) and Pb (II) using chemically modified orange peel: equilibrium and kinetic studies, Solid State Science, 2012, 14, p. 202-210.
18. Akpomie K. G., Dawodu F. A., Adebowale K. O., Mechanism on the sorption of heavy metals from binary solution by a low cost montmorillonite and its desorption potential, Alexandria Engineering Journal, 2015, 54, p. 757-67.
19. Nwadiogbu J. O., Okoye P. A. C., Ajiwe V. I. E., Nnaji N. J. N., Hydrophobic treatment of corn cob by acetylation: kinetic and thermodynamic studies, Journal of Environmental Chemical Engineering, 2014, 2, p. 1699-1704.
20. Nimmala A. R., Lakshimipathy R., Sarada N. C., Application of citrullus lanatus rind as biosorbent for removal of trivalent chromium from aqueous solution, Alexandia Engineering Journal, 2014, 53, p. 969-975.
21. Akpomie K. G., Dawodu F. A., Efficient abstraction of Ni(II) and Mn(II) ions from solution onto an alkaline modified montmorillonite, Journal of Taibah University for Science, 2014, 8, p. 343-356.
22. Krishnani K. K., Xiaoguang M. X., Christodoulatos C., Boddu V. M., Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk, Journal of Hazadous Material, 2008, 153, p. 1222-1234.
23. Taffarel S. R., Rubio J., On the removal of manganese ions by adsorption unto natural and activated Chilean zeolites, Mineral Engineering, 2009, 22, p. 336-343.
24. Kumar A., Prasad B., Mishra I. M., Isotherm and kinetic studies for acrylic acid removal using powdered activated carbon, Journal of Hazardous Material, 2010, 176, p. 774-783.
25. Tsai W. T., Chen H. R., Removal of malachite green from aqueous solution using low cost chlorella based biomass, Journal of Hazardous Material, 2010, 175, p. 844-849.
26. Li Y., Xia B., Zhao Q., Liu F., Zhang P., Du Q., Wang D., Li D., Wang Z., Xia Y., Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin, Journal of Environmental Science, 2003, 23, p. 404 -411.
27. Yadav S. K., Singh D. K., Sinha S., Chemical carbonization of papaya seed originated charcoals for sorption of Pb (II) from aqueous solution, Journal of Environmental Chemical Engineering, 2014, 2, p. 9-19
28. Gupta V. K., Jain C. K., Imran A., Sharma M., Saini V. K., Removal of cadmium and nickel from wastewater using bagasse fly ash- a sugar industry waste, Water Resources, 2003, 37, p. 4038-4044.