Inhibitive potential of Datura stramonium leaf extract on the corrosion behavior of mild steel in 1M HCl acidic solution
Gbenga Olabode OKE1,
Ayotunde Olugbenga ALUKO1, Olajide Tunmilayo SANYA2*
1,Mineral and Petroleum Resources Engineering Department, Federal Polytechnic Ado Ekiti, Nigeria
2 Glass and Ceramic Technology Department, Federal Polytechnic Ado Ekiti, Nigeria
E-mail(s): 1 okenature2@yahoo.com; 2 alayrehoboth@gmail.com; 3 tunmilayo@gmail.com
* Corresponding author, +234 803 479 7204
Abstract
Datura stramonium leaf extract (DSLE), as corrosion inhibitor in 1M HCl acid solution was assessed using phytochemical screening, atomic absorption spectroscopy (AAS), gravimetric (mass loss), and electrochemical (Tafel and potentiodynamic polarization) methods. The extract was found to efficiently inhibit the corrosion process and the inhibition efficiency increased with increasing extract concentration (over 400% increases) at room temperature. The maximum inhibition efficiency of DSLE in 1M HCl was found to be 98.69% at 0.5g/l concentration. The potentiodynamic polarization results revealed that DSLE functioned as mixed-type inhibitor acting on both the anodic and cathodic sites (but predominantly anodic control). The AAS analysis shows that the concentration of Fe2+in the electrolyte decreases with increase in the extract concentration. The adsorption studies of the extract on the metal surface showed that there was a physiosorption process as the concentration increases and the chemisorption process is also prevalent. The adsorption studies fitted the assumptions of Temkin and Freundlich isotherm models.
Keywords
Adsorption mechanism; Corrosion Inhibition; Datura stramonium; Mild steel; Polarization
Introduction
Mild steel is known to be a very multipurpose ferrous alloy utilized for a wide range of applications because of its excellent combination of mechanical properties, ease of fabrication, excellent weldability, and low purchasing cost [1]. However, it has a low corrosion resistance (undergoes oxidation reactions) especially in acidic environments [2]. This has been a major cause for concern because of its strong use in the design of vessels, storage devices and other industrial accessories used in food, petro-chemical and chemical plants where acidic media are often utilized. Uncontrolled corrosion has the negative effects of causing rigidity weakening of the mild steel based installations and impoverishment of mechanical strength and ultimately degenerates into premature failure [3]. The use of inhibitors has been widely explored to mitigate against corrosion of mild steel exploited in acidic environments. Often, nitrogen, sulfur- or oxygen-containing compound have been used and proven to be good acid corrosion inhibitors. However, due to certain characteristic properties such as difficulty in solubility and toxicity of most of the above mentioned inorganic salts or organic compounds, awareness of their ecological and health threats has drawn researchers to explore more environmentally friendly inhibitor sources. Many extracts of common plants have been found useful in this regard – plants such as Rice husk, Aquilaria crassna, Prunus cerasus, Tithonia diversifolia, Jatropha curcas, Moringa oleifera, Murraya koenigii and Aloe vera [4-13] have been investigated and research efforts are still ongoing to source for high performance green inhibitor alternatives with optimum concentration [14, 15].
Datura stramonium plant is a household name in Nigeria, especially in the southwestern part. The green plant is also known as gegemu in the local language. It is acknowledged for its use as constituent in herbal remedies for treatment of fever, to relieve arthritis, such as rheumatism and gout, heart problems like palpitations and hypertension among others. Moreover, it is exploited as a relief for asthma symptoms [16] and an analgesic during surgery or bone-setting. It is also a powerful hallucinogen and deliriant, which is used entheogenically for the intense visions it produces. However, the tropane alkaloids responsible for both the medicinal and hallucinogenic properties could be fatally toxic if slightly higher amounts are used [17-19].
In the present study, the corrosion inhibition efficiency of Datura stramonium leaf extract in 1M hydrochloric acid solution is evaluated by mass loss, atomic adsorption spectrometric, potentiodynamic polarization measurements and the adsorption isotherms studies. However, its use as corrosion inhibitor of mild steel has not been extensive reported in the literature.
Material and method
Materials preparation
Mild steel plate with chemical composition shown in Table 1 was used for this study. The mild steel rod was mechanically cut to individual coupons, each of dimensions, 300mm length by 12 mm diameter. Each coupon was washed with ethanol, cleaned with acetone and allowed to dry in the air to remove grease, oil and dirt. Analar grade HCl and distilled water were used for the preparation of the electrolyte, while leaves of Datura stramonium were used as a source of corrosion inhibitor.
Table 1. Chemical composition of the mild steel
|
Element |
Fe |
C |
P |
Mn |
Cr |
Mo |
Si |
S |
Cu |
|
% Composition |
98.31 |
0.13 |
0.06 |
0.82 |
0.08 |
0.02 |
0.26 |
0.05 |
0.27 |
Preparation of Datura stramonium extract
The Datura stramonium leaves were sourced from Ado-Ekiti metropolis, Nigeria. The leaves samples were washed, oven dried at 90°C for 4 hours and pulverized using a electrically powered blender. About 20g of the pulverized leaves was weighed and soaked in 100ml of ethanol for 48 h. The mixture was then filtered to obtain the extract. The filtrates were furthered subjected to evaporation at 352K to leave the sample free of the ethanol. The stock solutions of the extract obtained were used in preparing different concentrations of the extract by dissolving 0.1, 0.2, 0.3, 0.4 and 0.5g of the Datura stramonium leaf extract (DSLE) in 1M of HCl. Similar methods of preparing aqueous plant extracts have been widely reported in literatures in recent years [2, 20, 21].
Phytochemical screening
The phytochemical constitution of the Datura stramonium leaves was evaluated following standard procedures (in accordance to [22]). Specifically, the tannin, cardiac glycosides, saponnin, terpenoids and flavonoids were determined as follows:
Determination of tannins
The test for tannins (Braymer’s test): 2ml of extract was treated with 10% alcoholic ferric chloride solution and observed for formation of blue or greenish colour solution.
Determination of saponin
The test for Saponins (Foam test): 2ml of extract was added to 6ml of water in a test tube. The mixture was shaken vigorously and observed for the formation of persistent foam that confirms the presence of saponins.
Determination of cardiac glycosides
The test for Cardiac glycosides (Keller Kelliani’s test) 5ml of extract was treated with 2ml of glacial acetic acid in a test tube and a drop of ferric chloride solution was added to it. This was carefully underplayed with 1ml concentrated sulphuric acid. A brown ring at the interface indicated the presence of deoxysugar characteristic of cardenolides. A violet ring may appear below the ring while in the acetic acid layer, a greenish ring may form.
Determination of flavonoid
The test for Flavonoid (Alkaline reagent test) 2ml of extract was treated with few drops of 20% sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on addition of dilute hydrochloric acid indicate the presence of flavonoid.
Determination of terpenoid
The test for terpernoids (Salkowki’s test): 1ml of chloroform was added to 2ml of each extract followed by a few drops of conc. sulphuric acid. A reddish brown precipitate produce immediately indicates the presence of terpenoids.
Weight loss measurement
The polished and pre-weighed mild steel square specimens were immersed in 100 ml test solution with 0.0 - 0.5g/l inhibitor maintained at ambient temperatures for all the experiments for 5 days (120h). The weight loss of each coupon was determined at every 24hr intervals by retrieving the test samples from the solutions cleaned with acetone and weighed. From the weight loss data collected corrosion rate (CR) was calculated from weight loss measurement in accordance with ASTM G31 standard.
CR (mmy) = 87,500W/Aρt (1)
where CR is corrosion rate (mmy), W
is weight loss (g), A is the area of the coupon in cm2, t is time
(h), and ρ is the density (g/cm3).
Inhibition efficiency (IE) and surface coverage (![]() ) were estimated using equations (2)
and (3) respectively.
) were estimated using equations (2)
and (3) respectively.
I.E.% = (1-CRinh/CRblank) ×100 (2)
where CRinh and CRblank correspond to the corrosion rate in the presence and absence of inhibitor respectively; I.E. is the inhibition efficiency.
The surface coverage (θ) was determined by using equation (3)
θ = (1-CRinh/CRblank) (3)
Mass loss method (ASTM standard)
The polished and pre-weighed mild steel square samples were immersed in 100ml of the blank/inhibitor solution for 5 days (120 hr). The weight loss of each coupon was determined at every 24 h intervals by retrieving the samples from the solution, cleaned with acetone and weighed (separate solution was perpared for the mild steel samples). The mass loss for each sample was evaluated by dividing its weight loss by the surface area of the coupon.
Atomic adsorption spectrometric analysis
Atomic adsorption analysis was performed using atomic adsorption spectrometer model bulk 200. This was required to determine the concentration of iron (II) ions in the HCl solution after weight loss measurements. The calibration curve of iron (II) ions was drawn before analyzing the electrolyte solution. All samples containing iron ions were diluted with distilled water to ensure that the concentrations of metal ions are within the range of the calibration curve.
Electrochemical measurements
The electrochemical corrosion studies of mild in various concentration of Datura stramonium extract in 1M HCl were carried out using AUTOLAB PGSTAT 204N instrument, piloted by Nova software. The electrochemical cell will be a conventional three-electrode cell comprising the mild steel sample as the working electrode, saturated silver/silver chloride as reference electrode and platinum rod as counter electrode. The working electrode was immersed in test solutions 1M HCl solutions with or without datura stramonium leaf extract (DSLE) for 30 minutes until a stable open circuit potential was achieved. The working electrode were prepared by attaching an insulated copper wire to one face of the sample using an aluminium conducting tape and cold mounting it with epoxy resin. The potentiodynamic polarization measurements were carried out carried using à scan rate of 1.0 mV/s at a potential initiated at -250mV to +250mV with respect to OCP. After each experiment, the electrolyte and the test sample was replaced. The linear Tafel segments of the anodic and cathodic curves were extrapolated to corrosion potential to obtain the corrosion current densities (Icorr) and corrosion potential (Ecorr) (Alaneme, Osasona, Okotete, Olusegun, & Donatus, 2016). The inhibition efficiency has been calculated by using respective corrosion current densities (iocorr and icorr) in place of corrosion rates (CoR and CR) in Eq. (2).
The inhibition effeciency is defined as
IE% = (iocorr - icorr / iocorr ) × 100 (4)
where iocorr and icorr are the corrosion current density values in the absence and presence of inhibitor, respectively.
The value of free energy change i.e Gibbs free energy (ΔGads) for the adsorption process can be evaluated from the equilibrium constant of adsorption using the equation:
ΔGads = -RTln (CH2O Kads) (5)
where CH2O = 1000g/l in the solution, R is the universal gas constant, T is the absolute temperature and Kads is the equilibrium constant of adsorption. The value of Kads was calculated from the intercept of the straight line. The equilibrium constant is related to the standard free energy of adsorption ΔGads given in Eq. 5. Values of ΔGads around -20KJ/mol or below depict physisorption mechanism and those of about -40KJ/mol or above result in covalent bonds through chemisorption mechanism [23-26].
Results and Discussion
Phytochemical screening
Table 2 shows the phytochemical constituents in the datura stramonium leaf extracts (DSLE). The result shows the presence of tannins, flavoniods among others. The presence of these compounds has been reported to enhance the inhibition of mild steel in aggressive chloride environment [6, 7, 27].
Table 2. Phytochemical constituents of Datura stramonium leaf extract (DSLE)
|
Phytochemical constituents |
Saponins |
Tannins |
Flavonoids |
Keller Killani |
|
R-HCl |
- |
+ |
+ |
+ |
Mass loss measurement
The results of the variation in mass loss of the mild steel substrates in the absence and presence of varying concentrations of Datura stramonium leaf extract, DSLE as a function of exposure time at room temperature and different extract concentrations are shown in Fig. 1. It can be observed from the figure that the mass loss increased with increase in exposure time. However, the addition of extracts in 1M HCl solution resulted in a significant reduction in the mass loss of the mild steel substrate while compared with the blank solution. In addition, the quantity of mass loss of mild steel in the 1M HCl solution is inversely proportional to the concentration of the DSLE. The reduction in mass loss with increase in concentration of the extract can be elucidated by the adsorption of phytochemical constituents present in the extract as shown in Table 2. Nnanna et al. [28] and Alaneme et al. [6,7] reported that the adsorption of such compounds on the metal surfaces result in the formation of a surface layer barrier for charge and mass transfer between the base metals and the corrosive environments. Furthermore, the reduction in mass loss with increase in extract concentration could be attributed to the fact that as the active sites of the DSLE were increased, they combine with the corrosion products to form passive film layer on the coupon, thus protecting the mild steel from further attack by the acid [4]. Therefore, Datura stramonium leaves extracts could efficiently serve as an organic inhibitor of corrosion of mild steel immersed in 1M HCl environment.
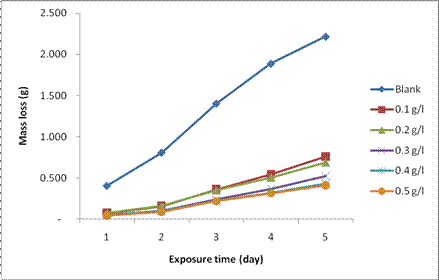
Figure 1. Plot of mass loss for the corrosion of mild steel in 1M HCl as a function of time in the absence and presence of DSLE
Atomic absorption spectroscopic analysis
Figure 2 presents the result of the atomic absorption spectroscopic analysis which shows the concentration of iron (II) ions dissolve in 1M HCl in the presence and absence of Datura stramonium leaf extract. The result obtained concurs completely with the mass loss measurement. It is seen that the concentration of iron (II) ions dissolved in the electrolyte decreased as the concentration of DSLE increases. This infers that there is an interaction that would have led to the adsorption of the extracts on the metal surface, thereby retarding the oxidation of iron to iron (II) ions [20, 21].
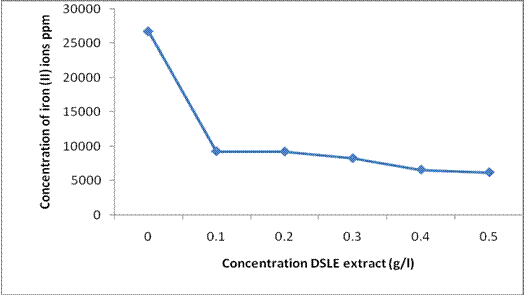
Figure 2. Plot of the concentrations of iron (II) ions in 1M HCl acid as a function of different concentration of DSLE
Electrochemical measurement
Polarization Studies
The potentiodynamic polarization curves and data for MS in 1M HCl acid solution in the absence and presence of different concentrations of DSLE at room temperature are presented in both Table 3 and Figure 3. Polarization studies was carried out to distinguish the effects of DSLE on the anodic dissolution and cathodic hydrogen evolution reaction of MS respectively. Electrochemical kinetic parameters such as corrosion potential (Ecorr), cathodic and anodic slope (βc and βa), corrosion current density (icorr) and percentage inhibition (IE%) were obtained from Tafel curves and are given in Table 3. The Table 3 shows that the icorr values decreased significantly in the presence of inhibitor than in the acid solution (blank) which was due to the increase in the blocked fraction of the metal surface by adsorption. This serves as a barrier between the aggressive medium and the mild steel, thereby reducing the loss of material to the acidic solution. Hence, the increase in inhibitor concentration reduces the rate of anodic dissolution [23]. This is in agreement with the results obtained for mass loss.
However, the visual observation of polarization curves, as shown in Figure 3, indicates that the value of corrosion potentials shift toward more negative direction with the highest inhibitor concentration of 0.5g/l, an indication of the affinity of the organic inhibitor for cathodic reaction [29]. Here the cathodic reaction is retarded on the addition of inhibitor and should be defined as cathodic inhibitor. Thus both anodic and cathodic reactions were suppressed with the addition of increase in DSLE concentration. This implies that the plant extract functioned as a mixed-type inhibitor (but predominantly of anodic control) [15].
Table 3. Polarization data of MS in 1M HCl solution in the absence and presence of different concentrations of DSLE at room temperature
|
Conc, Cinh (g/l) |
Ecorr (mV) |
icorr (uAcm-2) |
βa (mVdec-1) |
-βc (mVdec-1) |
CR (mm/yr) |
% IE |
|
Blank |
-451.192 |
141.126 |
84.715 |
148.58 |
1.63760 |
- |
|
0.1 |
-444.103 |
110.798 |
104.503 |
107.321 |
1.28570 |
21.49 |
|
0.2 |
-536.964 |
105.96 |
639.83 |
622.015 |
0.001229 |
24.92 |
|
0.3 |
-442.221 |
53.00 |
881.659 |
1160.0 |
0.000615 |
62.44 |
|
0.4 |
-471.732 |
20.73 |
571.029 |
516.995 |
0.000241 |
85.31 |
|
0.5 |
-668.921 |
1.84 |
710.28 |
164.819 |
0.000021 |
98.69 |
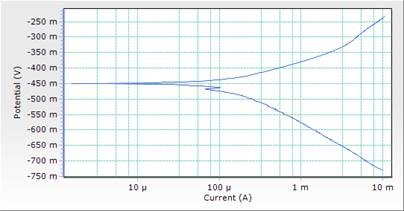
Figure 3. Comparison plot of cathodic and anodic polarization scans for mild steel in 1M HCl solution in concentrations of DSLE (0.0g/l)
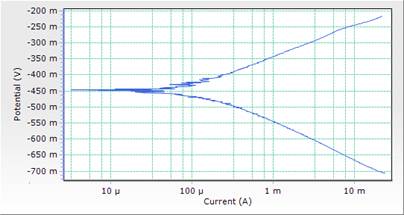
Figure 4. Comparison plot of cathodic and anodic polarization scans for mild steel in 1M HCl solution in concentrations of DSLE (0.1g/l)
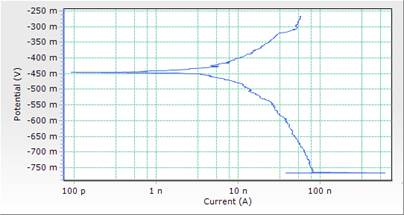
Figure 5. Comparison plot of cathodic and anodic polarization scans for mild steel in 1M HCl solution in concentrations of DSLE (0.3g/l)
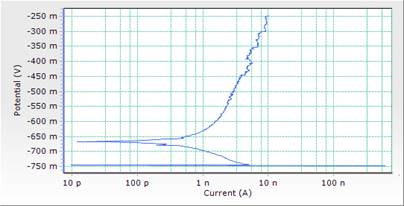
Figure 6. Comparison plot of cathodic and anodic polarization scans for mild steel in 1M HCl solution in concentrations of DSLE (0.5g/l)
Inhibition efficiency
The values of IE% were calculated from the potentiodynamic polarization measurements. It is observed that the inhibition efficiency increased with increase in the inhibition concentration (Table 3). The inhibition efficiency of 21.49% was observed for 0.1g/l DSLE concentration whereas for 0.5 g/l concentration, the highest efficiency of 98.69% was obtained in comparison (over 400% increase). As the DSLE concentration increases, the barrier film is formed on the MS surface which is attributed to the extent of adsorption and the resultant coverage by the DSLE inhibitor on the MS surface [15]. As the concentration of the DSLE increases, the interaction between the extract molecules and the mild steel samples would have increased thereby leading to a protective film on the surface [20,21].
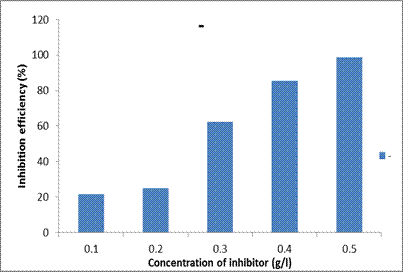
Figure 7. Inhibition efficiencies of different concentrations of Datura stramonium leaf extracts in 1M HCl acid solution
Table 4. Adsorption parameters for the adsorption ethanolic extract of DSLE on the surface of mild steel in 1M HCl at room temperature
|
Conc, Cinh (g/l) |
Ɵ |
C/Ɵ |
log Ɵ |
log C |
log C/Ɵ |
|
Blank |
- |
- |
- |
- |
- |
|
0.1 |
0.2149 |
0.4653 |
-0.6678 |
-1.0000 |
-0.3323 |
|
0.2 |
0.2492 |
0.8026 |
-0.6035 |
-0.6990 |
-0.0955 |
|
0.3 |
0.6244 |
0.4805 |
-0.2045 |
-0.5229 |
-0.3183 |
|
0.4 |
0.8531 |
0.4689 |
-0.0690 |
-0.3979 |
-0.3289 |
|
0.5 |
0.9869 |
0.5066 |
-0.0057 |
-0.3010 |
-0.2953 |
Adsorption isotherm
A mathematical estimation of the adsorption modes of the inhibitor concentration was carried out in order to have a good grasp of the adsorption mechanism involved in Datura stramonium leaf extracts, DSLE on mild steel. This behaviour of corrosion inhibitor extract provides in-depth assessment of the metal-inhibitor interaction [23,30]. The adsorption mode predominant in the study will depend on the factors such as the composition of the inhibitor and the nature of the surface charge on the metal. If the metal surface charge Ɵ is negative, the adsorption of the cations will be favoured, whereas anions adsorption is favoured in a positive surface charge. The degree of surface coverage (Ɵ) obtained from potentiodynamic polarization techniques was used to evaluate the best isotherms that fits into the data obtained [2, 15].
Correlation coefficient obtained from the plots of various isotherms such as Langmuir, Temkin, Freundlich etc. were used to determine the best isotherms most applicable to the experimental data.
From the correlation coefficient obtained, Temkin and Freundlich were found to be best applicable.
The Temkin adsorption isotherm is applied to investigate the mechanism of the corrosion inhibition by the following equation
Ɵ = 1/f (ln KadsC) (6)
Equation 6 implies that
Ɵ = 1/f ln Kads + 1/f ln C (7)
where C is the inhibitor concentration (g/l), Kads is the adsorption-desorption equilibrium constant, 1/f is the slope of the graph and Ɵ is the surface coverage which is given as:
Ɵ = % IE/100 (8)
A straight line was obtained on plotting Ɵ against log C (Temkin) which indicated a linear plot with a correlation coefficient of (R2 = 0.9331) and a slope (1.2526). The plot (Figure. 8) of surface coverage (Ɵ) against the natural logarithm of the concentration shows the correlation that adsorption energy decreases linearly with increase in the value of the surface coverage (Ɵ). The Temkin isotherm is often used for the description of chemisorptions models [31]. Theoretically, it is described by the relation given in equations (6) and (7).
The experimental data obtained was also fitted to Freundlich adsorption model using equation (9) given by:
log Ɵ = log Kf + 1/n log C (9)
where C is the inhibitor concentration (g/l), Kf is the adsorption-desorption equilibrium constant, n is the adsorption intensity and Ɵ is the surface coverage.
A linear graph was also obtained on plotting log Ɵ against log C (Freundlich isotherm). The plot is presented in Figure. 9. The slope of the graph is 1/n which is the function of the strength of adsorption in the adsorption process and log Kf is the intercept of the straight line. If 1/n = 1, the participation between the two phases is independent of the concentration. If 1/n is below 1, indicates a normal adsorption and depended on concentration and 1/n is greater than 1, it is indicative of cooperative adsorption. The values of 1/n is determined from the plot and is observed to be 1.0429. Since the value of 1/n is greater than 1, then it is indicative of cooperative adsorption [32, 33]. Freundlich isotherm states that the relationship between the amount and concentration of DSLE adsorbed onto the MS varies at different concentrations [23, 34]. The slope obtained is near unity and the correlation coefficient (R2 = 0.945) also confirms the isotherm into the experimental data. These indicators confirm that there was a higher surface adsorption process (physiosorption process).
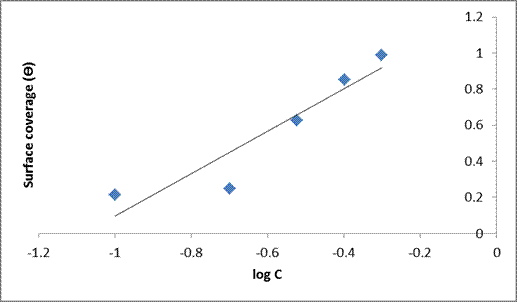
Figure 8. Temkin isotherm plot for mild steel in 1M HCl in the absence and presence of the varying extracts of DSLE at room temperature
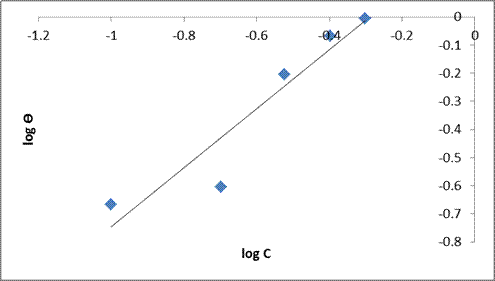
Figure 9. Freundlich isotherm plot for mild steel in 1M HCl in the absence and presence of the varying extracts of DSLE at room temperature
Thermodynamic evaluation of the corrosion process
The value of ΔGads that was determined from the Freundlich isotherm plot is -14.19KJ.mol-1, an indication that the value of ΔGads for the adsorption of DSLE on mild steel surface in 1M HCl reflects strong physical adsorption which is predominant coupled with slight chemisorption mechanism. The negative value of ΔGads also showed that the adsorption of DSLE is spontaneous.
Conclusions
The analysis of the inhibition potential of Datura stramonium leaf extract (DSLE) on the corrosion of mild steel in 1M HCl solutions at room temperature showed that:
· DSLE is a potential adsorption inhibitor for the corrosion of mild steel.
· The presence of tannins and flavonoids are responsible for the inhibitive potential of the extracts.
· The inhibition efficiency increases with increase in concentration of extracts with a maximum value (98.69%) at an optimum concentration (0.5g/l).
· The adsorption mechanism follows the Temkin and Freundlich isotherms proving that as the inhibitor concentration increases, there is higher surface adsorption process.
· The polarization studies showed that DSLE functioned as a mixed-type inhibitor by acting on both the anodic and cathodic sites (but more of anodic control).
References
1. Yaro A. S., Khadom A. A., Wael R. K., Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid, Alexandria Eng. J., 2013, 52 (1), p. 129-135.
2. Muthukrishnan P., Jeyaprabha, Prakash P., Mild steel corrosion inhibition by aqueous extract of Hyptis suaveolens leaves, International Journal of Industrial Chemistry, 2014, 1, Article 5, p. 1-11.
3. Ahmad Z., Principles of Corrosion Engineering and Corrosion Control, Butterworth-Heinemann (Elsevier imprint), UK, 2006.
4. Alaneme K. K., Olusegun S. J., Corrosion Inhibition performance of lignin ettract of sun flower (Tithonia diversifolia) on medium carbon low alloy steel immersed in H2SO4 solution, Leonardo J. Sci., 2012, 20 (11), p. 59-70.
5. Olusegun S. J., Adeiza B. A., Ikeke K. I., Bodunrin M. O., Jatrophas Curcas leaves extract as corrosion inhibitor for mild steel in 1M hydrochloric acid, J. Emerging Trends Eng. Appl. Sci., 2013, 4(1), p. 138-143.
6. Alaneme K. K, Daramola Y., Olusegun S., Afolabi A., Corrosion inhibiton and absorption characteristics of rice husk extracts on mild steel immersed in 1M H2SO4 and HCl solutions, International Journal of Electrochemical Science, 2015, p. 3553-3567.
7. Alaneme K. K, Olusegun S., Adelowo O., Corrosion inhibition and absorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions, Alexandria Engineering Journal, 2015, p. 1-9.
8. Helen L. Y. S., Rahim A. A., Saad B., Saleh M. I., Bothi Raja P., Aquilaria crassna leaves extracts - a green corrosion inhibitor for mild steel in HCl medium, Int. J. Electrochem. Sci., 2014, 9, p. 830-846.
9. Ashassi-Sorkhabi H., Seifzadeh D., The inhibition of steel corrosion in hydrochloric acid solution by juice of Prunus cerasus, Int. J. Electrochem. Sci., 2006, 1, p. 92-98.
10. Singh A., Ahamad I., Yadav D. K., Singh V. K., Quraishi M. A., The effect of environmentally benign fruit extract of shahjan (Moringa oleifera) on the corrosion of mild steel in hydrochloric acid solution, Chem Eng. Commun., 2012, 199 (1), p. 63-77.
11. Beenakumari K. S., Inhibitory effects of Murraya koenigii (curry leaf) leaf extract on the corrosion of mild steel in 1M HCl, Green Chem. Lett. Rev., 2011, 4 (2), p. 117-120.
12. Abiola O. K., James A. O., The effects of Aloe Vera extract on corosion and kinetics of corrosion process of zinc in HCl solution, Corros. Sci., 2010, 52(2), p. 661-664.
13. Mehdipour M., Ramezanzadeh B., Arman S. Y., Electrochemical noise investigation of Aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H2SO4, J. Ind. Eng. Chem., 2015, 21, p. 318-327.
14. Ji G., Anjum S., Sundaram, Prakash R., Musa paradisiac peel extract as green corrosion inhibitor for mild steel in HCl solution, Corros. Sci., 2015, 90, p. 107-117.
15. Mourya P., Banerjee S., Singh M. M., Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor, Corros. Sci., 2014, 85, p. 352-363.
16. Guharov S. R., Barajas M., Intense Stimulant Effect atropmeintoxication from the ingestion and smoking of Jemson weed (Datura Stramonium), Vet. Toxicol., 1991, 33, p. 588-589.
17. Banso A., Adeyemi S., Phytochemical Screening and Antimicrobial Assessment of Abutilon Mauritianium, Bacopa Monnifera and Datura Stramonium, Biokemistri, 2006, 18(1), p. 39-44.
18. Konkwara J. O., Medicinal Plants of East Africa, Literature Burea, Nairobi, 1976, pp. 7-8.
19. Fluck H., Medicinal Plants and their uses, W Feul Shom and Comp, Ltd, New York, 1973, pp. 7-15.
20. Alaneme K. K, Olusegun S., Alo A., Corrosion inhibitory properties of elephant grass (Pennisetum purpureum) extract on mild steel corrosion in 1M HCl solution, Alexandria Engineering Journal, 2016, p. 1-8.
21. Alaneme K. K., Osasona B., Okotete E., Olusegun S., Donatus U., Corrosion inhibition behaviour of Biden pilosa extract on aluminium matrix composites in 1 HCl solution, The Journal of the Association of Professional Engineers of Trinidad and Tobago, 2016, 44(2), p. 35-42.
22. Trease G. E., Evans W. C., Pharmacology, 13th ed. Bacilliere Tindal, London, 1991, pp. 60-75.
23. Loto R. T., Loto C, A., Popoola A. P. I., Fedotova T., Inhibition Effect of butan-1-ol on the Corrosion Behaviour of Austenitic stainless steel (Type 304) in dilute Sulphuric acid, Arabian Journal of Chemistry, 2015, doi: 10.1016/J.arabjc. 2014.12.024
24. Deng S., Li X., Inhibition by Jasmine nudiflorum Lindl leaves extract of the corrosion of aluminium in HCl solution, Corros. Sci, 2012, 64, p. 253-262.
25. Amin M. A., Ibrahim M. M., Corroson and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative, Corros. Sci., 2011, 53, p. 873-885.
26. Umoren S. A., Obot I. B., Ebenso E. E., Corrosion inhibition of aluminium using exudates gum from Pahylobus edulis in the presence of halide ions in HCl, E-Journal of Chemistry, 2008, 5(2), p. 355-364.
27. Olusegun S. J., Oluwasina O. O., Alaneme K. K., Olubambi P. A., Corrosion inhibition of mild steel in acidic solution by cow dung extracts as an eco-friendly inhibitor, J. Mater. Environ. Sci., 2016, 7(4), p. 1086-1097.
28. Nnanna l., Nnanna G., Nnakaife J., Ekekwe N., Eti P., Aqueous extracts of Pentaclethra Macrophylla Bentham Roots as Eco-friendly Corrosion inbition of mild steel in 0.5M KOH medium, International Journal of Materials Chemistry, 2016, 6(1), p. 12-18.
29. Li W. H., He Q., Zhang S. T., Pei C. L., Hou. B. R., Some New Triazole derivatives as Inhibitors for Mild Steel Corrosion in Acidic Medium, J. Appl. Electrochem., 2008, 38(3), p. 289-295.
30. Quraishi M. A., Ansari F. A., Jamal D., Corrosion Inhibition of Tin by Some amino acids in Nitric acid solution, Ind. J. Chem. Tech., 2004, 11(2), p. 271.
31. Oguzie E., Corrosion Inhibitive Effect and Adsorption behaviour of Hibiscus sabdariffa extract on Mild Steel in Acidic Media, Port. Electrochim. Acta., 2008, 26, p. 303-314.
32. Olusegun S., Okoronkwo E., Okotete A., Ajayi O., Gravimetric and electrochemical studies of corrosion inhibiton potential of acid and ethanol extract of siam weed on mild steel, Leonardo Journal of Science, 2016, 24-42.
33. Dada A. O, Olalekan A. P., Olatunya A. M., Dada O., Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk, Journal of Applied Chemistry (IOSR-JAC), 2012, 3(1), p. 38-45.
34. Unuabonah E. I., Olu-Owolabi B. I., Adebowale K. O., Ofomaja A. E., Adsorption of lead and cadmium ions from Aqueous solutions by Tripolyphosphate-impregnated Kaolinite Clay, Coloids Surf. A: Physicochem. Eng. Aspects, 2007, 292(2-3), p. 202-211.