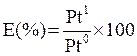Optimization of the hydrolysis cake filtration process in platinum extraction
Duke PHAHLA1, Abraham Adewale ADELEKE2, Abimbola Patricia POPOOLA1, and Babs Victor OMIDIJI3,*
1Department of Chemical, Metallurgical and Materials Engineering, Tshwane University of Technology (TUT), Pretoria, Private Bag X680, South Africa
2 Department of Materials Science and Engineering, Obafemi Awolowo University, PMB 22005, Ile Ife, Nigeria
3Department of Mechanical Engineering, Obafemi Awolowo University, PMB 22005, Ile Ife, Nigeria
E-mail(*): bvomidiji@gmail.com
* Corresponding author, phone: +234 8035728294
The study was carried out to optimize the filtration of the hydrolysis cake obtained during the refining stage of platinu-rich liquor. The platinum refining stage cake was first filtered in the glove box and secondly in the filter press to optimize the recovery of platinum value. The results obtained indicated that the filter press operation gave the highest and lowest platinum recovery efficiencies of about 99.95 and 97.69% as against 99.39 and 97.21%, respectively, for the glove box. In addition, the trend lines obtained showed that the filter press operation results following almost a linear trendline as being more stable than the glove box with a steeper trendline. Furthermore, the filter press processing of the hydrolysis cake took only about five hours in comparison to about 14 hours for the glove box. The filter press has thus proven to be a more efficient method to optimize platinum recovery from hydrolysis cake.
Keywords
Platinum; Hydrolysis; Filter; Press; Efficiency
Introduction
The extraction of platinum group metals and other precious metals from the converter matte involves smelting where the original matte is broken down to yield metallic nickel and copper anodes, an electrorefining step in which the anodes are electrolytically dissolved to produce nickel and copper and lastly chemical operations for the separation and refining of the individual PGMs [1-3]. In the first stage of the chemical operation to extract platinum from its liquor, the liquor is dissolved and hydrolysed to produce a cake [4, 5]. The hydrolysis cake produced is then taken to a vacuum filtration process that uses a glove box to form platinum richer liquor leaving a dry residue. However, the inefficiency of the glove box makes the hydrolysis cake residue formed in this process to contain high moisture with platinum entrainment necessitating a seven stage re-processing. In view of the current scarcity of high quality ore feed and the high cost of mining and mineral processing, the old and slow glove box filtration technology is no longer efficient to keep up with the modern operational requirement. Figure 1 shows the platinum extraction process based on the glove box technology and starting with platinum rich liquor [6, 7].
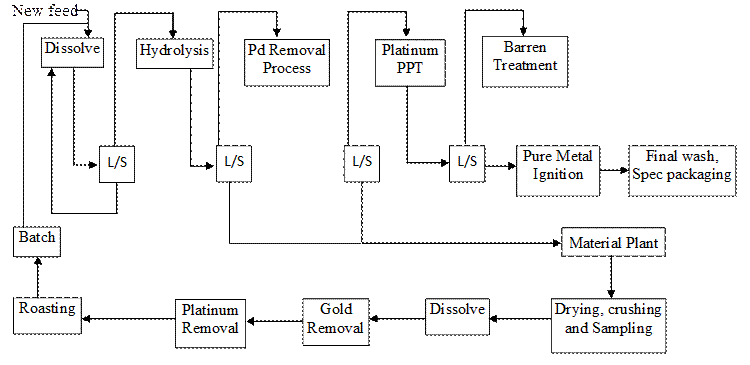
Figure 1. Flow chart of platinum extraction process
The glove box is normally dressed with a needle-felt cloth and filter papers that are replaced after every single filtration process of a batch at a high cost. The entrained platinum in the hydrolysis cake and filter paper is normally transferred for drying in gas powered calcining ovens at temperatures of 1000oC. The dried residues are batched up and re-introduced again into the plant for further processing on the circuit that handles the impure liquor for up to 3 days. The platinum recovered from this process is then sent to the platinum ignition ovens for roasting at a temperature above 800oC. The roasted platinum-rich residue is batched up again to be fed to the platinum circuit where about 2.8% of the platinum in the batch will report to the hydrolysis residue again [8]. The platinum bearing samples are typically analysed for platinum, palladium, rhodium and iridium by Inductively Coupled Plasma Mass Spectrometer and atomic emission spectroscopy [9-11]. The laboratory methods to determine moisture and soluble salts content in soil and rock by mass has been elaborated in ASTM D2216-10 [12]. The tests were carried out in the plant based on ASTM D2216-10 [12] and the project student only provided the specific details he was permitted to give.
Cost-effective solid-liquid separation technology is essential for the process of de-watering base metal and precious metal concentrates. The selected technology is expected to achieve the target process parameters (such as final moisture content and solids throughput rate), to operate reliably in harsh environments and to provide low installation, operating, and maintenance costs [13]. Filtering units have evolved over the years with the introduction of better and more efficient filtering units. One of the widely used modern filtering technologies in the platinum processing industry includes pressure filtering. Most pressure filters are designed with actuated filter plates and whether the filter plates are arranged vertically or horizontally, the filter plates are mechanically linked to open and close in sequence usually by a hydraulic cylinder. Once closed, chambers are formed between the filter plates. Slurry is pumped into the chambers and solids are retained on the filter cloth inside the chamber and form a filter cake [14-18]. The solids are consolidated either under pump pressure and/or by a rubber membrane, which is inflated with compressed air or pressurized water. After consolidation, the solids can be further dried by forcing compressed air to flow through the filter cakes. The majority of the pressure filters installed for de-watering platinum group metal (PGM) concentrates utilize membrane technology to consolidate and de-water the filter cake. The pneuma filter press was developed by Rio Tinto South Africa as result of problems experienced with liquids/solids separation over the years. The introduction of the pneuma filter press, a remarkably simple filtration system greatly improved solids and filtrate production, while significantly reducing the cost of operation. The installations of finer grinding facilities in the platinum processing plants and the greater difficulties associated with the drying of finer ores makes the replacement of the less efficient glove box technology more urgent [19, 20].
The aim of this study was to investigate the filtration process of the platinum liquor hydrolysis and to optimize it in order to reduce cycle times and platinum entrained in the hydrolysis cake residue.
Materials and Methods
The stream of platinum rich liquor was split into two reactors of 200 litres each to accommodate high volumes of reagents to be added for the hydrolysis process.
The hydrolysis process
The flow diagram of the hydrolysis process in the glove box is presented in Figure 2.
Figure 2. Flow diagram of the hydrolysis process in the glove box
The density and pH of the liquor were adjusted and precious metal impurities such as Pd were removed by adding oxidizing and reducing agents. The liquor was cooled down to determine the concentration of the impurities. When the desired levels of impurity residues were achieved, the liquor was cooled down again in preparation for filtration. After filtration, the cake was removed and re-processed further in another plant to recover the Pt entrained in the hydrolysis cake. If the sample contained other precious metals concentration of >10 ppm, it was treated further using the hydrolysis process to precipitate the impurities. The solution containing hydrolysis cake and a platinum rich liquid was the input material fed to the glove box and filter press for the de-watering processes.
Glove box drying
The hydrolysis cake containing about 1.8 kg of impurities was dropped into the glove box (GBA) and filtered by vacuum for solid/liquid separation. The cake was filtered through four filter papers and a needle felt cloth. The vacuum system was left open overnight for 8 hours to ensure optimum cake drying. The filter paper and the cake weighing about 3.8 kg were removed in the morning and processed further in the materials department. The glove box filtration took about 14 hours. Before the residue cake was prepared for sampling, it was roasted in a muffle oven for about 7 hours and crushed in a hammer mill to 500 µm. The roasted sample was placed on a 10-way rotary sample divider and sub-split into another 10 samples. Accuracy was determined by random analysis of two cup samples. The boil down reduction losses (BORL) tests was conducted to quantify the moisture and soluble salts in the cake. The procedure described was repeated with 14 other batches.
Filter press drying
The hydrolysis process product with its impurities was filtered through the filter press (FPA) having cloths lined with a filtering aid, with the reactor’s contents at room temperature to prevent thermal shock on filter plates. The facility was operated at high pressure with the washing and drying steps using compressed air. The feed was allowed to pass through the filter press and the filtrate was recycled to the feed tank in order to determine if the filtrate was free of solids. The return valve was closed when the filtrate had no more solids and it was pumped forward to the next process. The filter press filtration took about 5 hours. The solid residue obtained was pre-squeezed and washed with process water. The cake residue was further air dried for 5 hours. The cake roasting and sample preparation was treated the same way as that of the glove box. The efficiency of the filtration was determined with the following formula:
|
|
(1) |
where, E is the efficiency of the process in %, Pt0 and Pt1 are platinum content (g/t) in the cake before filtration and the content of the filtrate recovered after filtration, respectively.
Results and discussion
The results obtained for the platinum contents of the cake residue of the filter press (FPA) and glove box (GBA) filtrations are presented in Figure 3. The obtained results showed that in general, the Pt contents of the cakes obtained in both cases vary for all the samples but with notable peaks at 1, 6, 17, 30 and 1, 5, 27, 29 for glove box and filter press cakes, respectively. The highest and lowest Pt contents of 1.8 and 0.324 g/t were obtained at the 1st and 22nd samples for the glove box, while at 0.696 and 0.043 at 29th and 10th samples for the filter press. More over, for all the samples, the glove box cakes have higher contents of Pt with its lowest value of 0.043 far lower than the 0.324 for the glove box. The results obtained thus indicated that the filter press performed more efficiently than the glove box by retaining lesser contents of Pt in its cake.
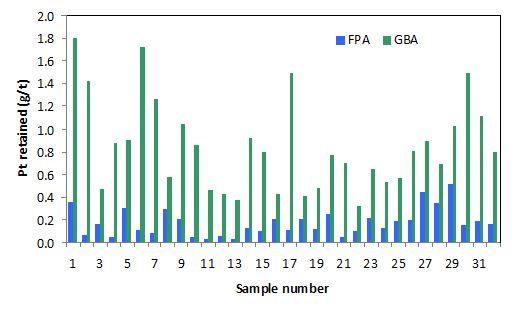
Figure 3. The Pt (g/t) retained in the filter press (FPA) and glove box (GBA) after the filtrationof the hydrolysis cake
Figure 4 shows the Pt retained in the filter press cakes (FPAP) and glove box cakes (GBAP) as percents of the Pt content of the corresponding input batch. The results showed that the glove box cakes generally have higher percentages of Pt retained than the filter press at all corresponding input batches with its highest percent of 0.31 occurring at sample 26 and that for filter press being 0.30 at 27th sample. In general, the percents of the batch Pt retained in both filters have no clear pattern of relationship with the Pt content of the input batch, but the most notable peaks in both cases appear between samples 18 and 27. This observation suggests that the filtering process of the samples in this range was less efficient and needs to be investigated.
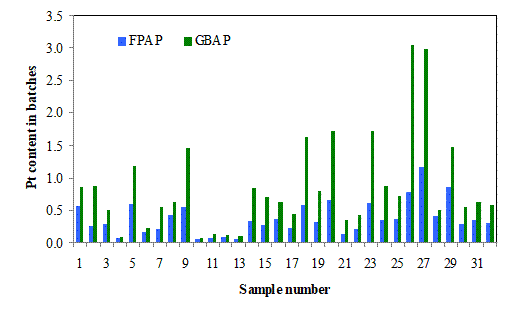
Figure 4. Pt contents of the filter press and glove box cakes in % (×10) of batch inputs
Figures 5(a) and 5(b) show the Pt contents in g/t of the batch input for filter press (FPA) and glove box (GBA). The Pt contents of the batch inputs vary randomly with the highest of 85.91 g/t for the 10th sample and the lowest of 25.66 g/t for the 26th sample. At the lowest grade of batch input, the Pt retained were 0.20 and 0.80 g/t for the filter press and glove box; while at the highest grade it was0.043 and 0.86 g/t, respectively. The results showed that the highest grade batch input yieled lower Pt retained in the filter press in comparison to the lowest grade, but the effect on the glove box appears negative. The highest values of the Pt entrained in the filter press and glove box of 0.51 and 1.8 g/t occurred at the samples 29th and 1st, respectively. The two points for the highest retained in the two filters occurred at 58.84 and 64.68 g/t of Pt in batch inputs. Thus, there is no clear pattern of relationship between batch inputs and Pt retained in both filtrations. The results obtained further suggest that the value of the Pt retained in cakes in both cases has no clear relationship with the Pt contents of the batch input.
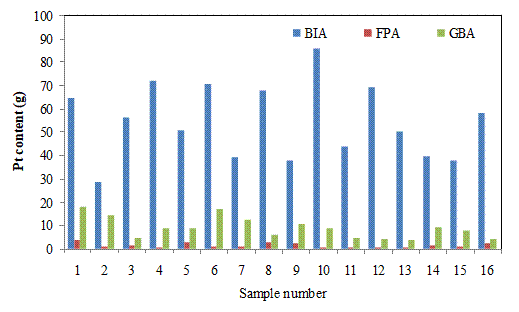
Figure 5(a). Pt contents of the batch, filter press cake (×10) and glove box cake (×10) (BIA=batch inputs, FPA =filter press, GBA= glove box)
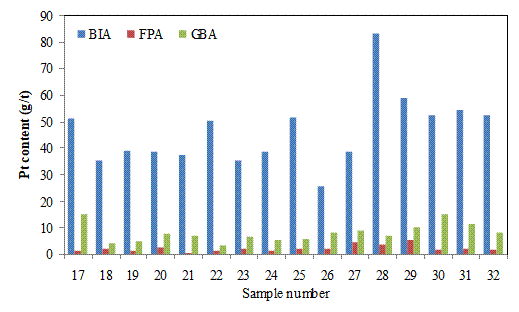
Figure 5(b). Pt contents of the batch, filter press cake (×10) and glove box cake (×10) (BIA=batch inputs, FPA =filter press, GBA= glove box)
Figure 6 shows the Pt recovery efficiency percents for the filter press (FPE) and the glove box (GBE) and the trend lines. The results showed that the filter press was more efficient in the recovery of Pt from the hydrolysis cake for all the samples. The percents in both cases very randomly and the trend lines showed the filter press results with almost a linear trendline as being more stable than the glove box with a steeper trendline. The results obtained further indicated that the filter press operation gave the highest and lowest efficiencies of about 99.95 and 97.69% as against 99.39 and 97.21% for the glove box.The results obtained thus suggest the filter press operation produced less variance in the data obtained and thus more stable than that of glove box.
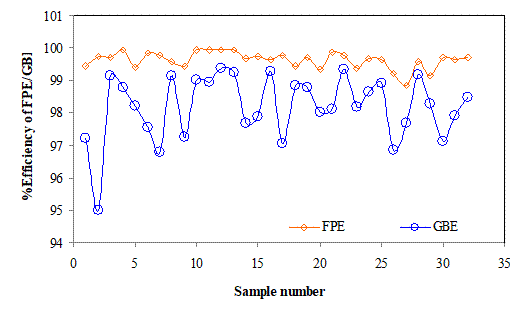
Figure 6. Efficiency of the filter press (FPE) and glove box (GBE) in treatment of hydrolysis batch inputs (BIA) with trend line
Figures 7(a), 7(b) and 7(c) show the Pt contents of the batch cake inputs (BIA) and the outputs of filter press (BOFP) and glove box outputs (BOGB) and their corresponding standard errors. The Pt contents in the three cases generally vary randomly with alternating increase and decrease. This fluctuations in the Pt contents may be due to batch splits as a results of high volumes generated during the hydrolysis proces.The standard errors obtained in the three cases for each data are generally similar suggesting that the platinum content determinations were of good precision.
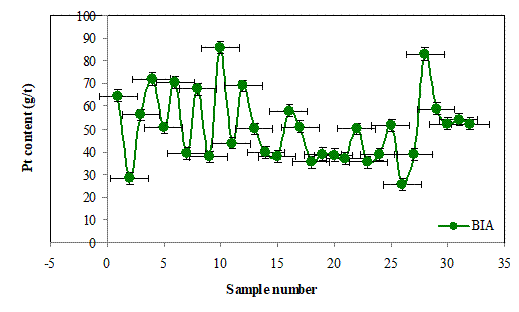
Figure 7. (a) The Pt (g/t) contents of hydrolysis batch cakes inputs (BIA) and standard errors
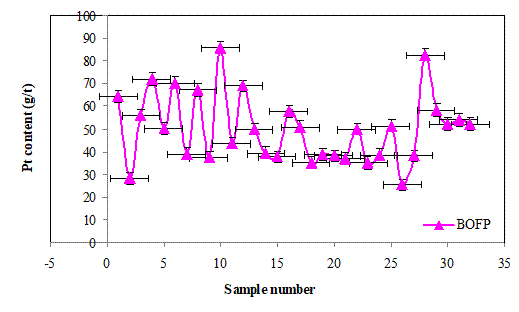
Figure 7. (b) The Pt (g/t) contents of filter press outputs (BOFP) and standard errors
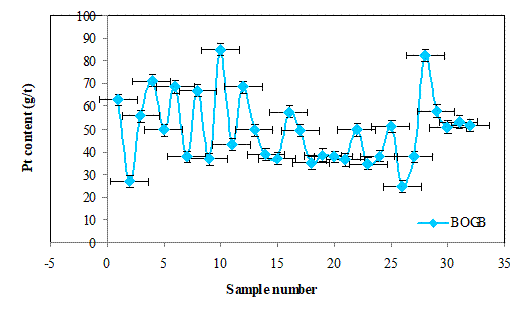
Figure 7. (c) The Pt contents of glove box outputs (BOGB) and standard errors
Conclusions
The filter press filtration carried out at high pressure with washing and drying done under compressed air has proved to be a more efficient de-watering system than the glove box. The filter hydrolysis cakes contained lower entrained platinum for all the samples and gave higher process efficiency than the glove box. In addition, the filter press was found to be more stable producing low variance in its data. It was also observed during the test work that the frequency of opening the filter press unit was much less compared to that of the glove box thus reducing the exposure of employees to the irritating airborne platinum salts. The filtration cycle time was also found to reduce from over 14 hours for glove box to less than 5 hours for the filter press.
References
1. Gouldsmith A. F. S., Wilson B., Extraction and Refining of Platinum Metals, A Complex Cycle of Smelting, Electrolytic and Chemical Operations, Platinum Metallurgical Revision, 1963, 7(4), p. 136-143.
2. Charlesworth P., Separating the Platinum Group Metals by Liquid-Liquid Extraction: New process has economic advantages over conventional selective precipitation, Platinum Metallurgical Revision, 1981, 25(31), p. 106-112.
3. Bezuidenhout G. E., Eksteen J. J., Refining of platinum group metals concentrates. Available at: https://encrypted.google.com/patents/EP2670871A1?cl=no, (accessed 18/12/2017)
4. Rosenqvist T., Extractive Metallurgy, Trondheim, Norway, Tapir Academic Press, 2012.
5. Downey J. P., Battle T. P., White J. F., International Smelting Technology Symposium: Incorporating the 6th Advances in Sulfide Smelting Symposium. Available at: https://books.google.com › Technology & Engineering › Materials Science › General (accessed 18/12/2017)
6. Lee J. Y., Raju B., Kumar B. N., Kumar J. R., Park H. K., Reddy B. R., Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst, Separation and Purification Technology, 2010, 73(2), p. 213-218.
7. Pospiech B., Studies of platinum recovery from solutions after leaching of spent catalysts by solvent extraction, Physicochemical Problems of Mineral Processing, 2012, 48(1), p. 239-246.
8. Habashi F., Principles of Extractive Metallurgy, Gordon & Breach Science Publisher, New York. 1970.
9. Taylor C. A., Howe A.M, Final report on project R48113 ICP-AES method for metals in air: Pt 2. Available at: http:www.hse.gov.uk/research/hsl_pdf/2002/hsl02-05.pdf (accessed 18/12/2017)
10. Linnemann U., The Evolution of the Rheic Ocean- From Avalonian-Cadomian Active Margin to Alleghenian-Variscan Collision, Geological Society of America. Available at: https://books.google.com.ng/books?isbn=0813724236 (accessed 18/12/2017)
11. Cooley E. F., Curry K. J., Carlson R. R., Analysis for the platinum group metals by fire-assay emission spectroscopy, Applied Spectroscopy, 1976, 30(1), p. 52-56.
12. ASTM D2216-10, Standard Test Methods for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass, Active Standard ASTM D2216. Developed by Subcommittee: D18.03 Book of Standards, Volume: 04.08
13. Pepper D., Rule C. M., Mulligan M., Cost-effective pressure filtration for platinum concentrates. The 4th International Platinum Conference, Platinum in transition ‘Boom or Bust’, The Southern African Institute of Mining and Metallurgy. Available at: www.flsmidth.com/~/media/Files/Papers/CostEffectivePressureFiltration.ashx (accessed 18/12/2017)
14. Persson W., Wendt W., Optical spectroscopy for process Monitoring and production control in ferrous and nonferrous industry in Materials Science and Technology -Modelling, Control and Optimization in Ferrous and Nonferrous Industry, Chicago, 2003, pp. 177-191.
15. Wills B. A., Mineral processing technology, 1997 Butterworth-Heinemann, Oxford
16. Mular A. L., Halbe D. N., Barrat D. J., Mineral processing plant design, practice and control Littleton, Society for Mining, Metallurgy and Exploration, 2002.
17. Ismail M. A., Eltayeb M. A. Z., Abdel Maged S. A., Elimination of Heavy Metals from Aqueous Solutions using Zeolite LTA Synthesized from Sudanese Clay, Research Journal of Chemical Sciences, 2013, 3(5), p. 93-98.
18. Khadse S., Panhekar Deepa, Patil Pralhad, Synthesis of Zeolite using Fly ash and its application in Removal of Cu2+, Ni2+, Mn2+ from Paper Industry Effluent, Research Journal Chemical Sciences, 2014, 4(3), p. 5-9.
19. Steenekamp N., Dunn G. M., Operations of and improvements to the Lonrho Platinum Base Metal Refinery, Warrendale, PA, The Minerals, Metals and Materials Society, 1999, pp. 365-378.
20. Smidth F. L., Cost effective solid-liquid separation. Available at: www.flsmidth.com/.../Liquid-Solid%20Separation/Filtration/Pneumapress/ChemShow (accessed 18/12/2017)