Morphology, FTIR and X-Ray patterns of pulverized mercerized Jack Bean (Canavalia ensiformis) seed coats
Abraham Olasupo
OLADEBEYE1*, Melodia Oshoke
OWARE2, Friday ODIA2, and Aderonke
Adenike OLADEBEYE3
1Department of Chemistry, University of Medical Sciences, Ondo, Nigeria.
2Department of Polymer Technology, Auchi Polytechnic, Auchi, Nigeria.
3Department of Food Technology, Auchi Polytechnic, Auchi, Nigeria.
* Corresponding authors: e-mail: oladebeye@gmail.com; phone: +234-803-3620-279
Abstract
The seed coats of jack beans (Canavalia ensiformis) were pulverized and modified via mercerization process. The chemical properties, morphology, Fourier-Transform InfraRed spectroscopy (FTIR) spectra and X-ray patterns of the unmodified and modified samples were studied. The unmodified sample was more acidic and hydrophilic than the mercerized sample. The iodine absorption number of the unmodified sample was 1.98 ml while the mercerized was 1.00 ml. The micrographs of unmodified sample showed irregular shapes of the granules while those of the mercerized derivative were mixes of cylindrical and rod-like shapes, which appeared more crystalline and fibrous than the unmodified. The mercerized sample showed (O-H) spectra of normal polymeric hydroxyl group, which had a characteristic signal of lignocellulosics. In addition, absorption band 2129.24 cm-1, indicating C≡C terminal alkynes (mono substituted), was found in the mercerized sample. Very strong NO of nitro group was observed at 1540.26 cm-1, which disappeared upon modification. The XRD profile showed peaks of both the modified and unmodified as mixes of A- and B- polymorphs, that is, C-type, with high proportion of silicone. The X-ray patterns were unchanged after modification. However, the crystallite size of the unmodified sample increased from 8.60 to 10.10. Therefore, the mercerized fiber were more applicable in the fabrication of fiber-polymer composites than the unmodified fiber.
Keywords
Jack bean; Pulverized; Modified; Unmodified; Patterns; Polymorphs; Iodine absorption; Morphology
Introduction
Vegetable fibers, which are a class of natural fibers, have attracted the attention of the composites community as a potential replacement of costly synthetic fibers [1, 2]. These natural fibers, in addition to low cost, are biodegradable, low dense, nonabrasive and readily available [3]. However, in their natural form, these natural fibers are affected by numerous problems, which revolve around the incompatibility between their hydrophilic nature and the hydrophobic nature of the polymer matrix, forming aggregates instead of composites [4]. Thus, their products exhibit high moisture affinity, poor mechanical properties, low thermal and chemical instability. To overcome these shortcomings, chemical modifications can either be done on the natural fiber, polymer matrix or both materials [5], aimed at lowering the hydrophilicity of the fiber, enhancing the interfacial bonding between matric and fiber and the overall mechanical properties of composites reinforced with natural fiber [6].
The commonest chemical modification of natural fiber is mercerization. Mercerization, which is an alkali treatment process of subjecting a vegetable fiber to an interaction with a fairly concentrated aqueous solution of strong base, alters the chemical architecture and behaviour of a natural fiber [7]. Cellulose, a vegetable fiber, has been reported to be greatly affected by mercerization, resulting in high swelling, which affects the crystallinity, morphology, thermal and mechanical properties [7]. As a matter of fact, the swelling degree of fiber during mercerization largely depends on the type and concentration of the alkali used [7]. Sodium hydroxide, owing to the tendency of sodium ions (Na+) to expand the pores of the lattice planes and penetrate, has been the most efficient alkaline used to achieve mercerization [8].
Obtaining natural fibers from various crops, especially the underutilized crops, is a way of meeting the increasing quest for possible replacement for synthetic fibers. Of paramount interest is unveiling the potentials of these underutilized agro-materials coupled with the possibility of turning green waste to useful raw materials, especially for non-food applications.
Jack bean (Canavalia ensiformis) seed, white in colour and nearly oblong in shape, is one of the neglected under-utilized legumes [9]. It is a tropical climber producing long pendant green bean, which belongs to the family of the leguminasae. Recently, the latent potentials of this tropical crop have been unveiled as a for various food and non-food applications [10, 11].
The present work was borne out of curiosity to know the chemical constituents of jack bean seed coat, which, hitherto, is considered as a waste, and turn it to a useful vegetable fiber. As at the time of this research, the authors are not aware of any article on the characterization of seed coats of jack bean as fiber.
This paper is aimed at: (i) isolating and pulverizing seed coats of jack beans (Canavalia ensiformis), (ii) modifying the pulverized seed coats via mercerization process, and (iii) characterizing both the unmodified and modified jack bean seed coat with a view to proposing its possible non-food applications.
Materials and methods
Sources of materials
Jack beans (Canavalia ensiformis) were freshly harvested from a farm in Auchi, Etsako-West Local Government Area, Edo State, Nigeria. All the reagents used were of analar grade.
Preparation of pulverized jack bean seed coat
The seed coats of jack beans were manually separated from the embryo, dried in direct sunlight for 4-6 days, followed by thorough manual removal of notable foreign materials such as dirts, broken cotyledons and immature seeds. The dry seed coats were pulverised in a Willey Mill (Scientific Equipment), sieved into a fine particle (250 µm) and the sample packaged in a transparent polythene bag prior to analysis.
Preparation of mercerized pulverized jack bean seed coat
50 g of the pulverized sample was soaked in 10% NaOH solution for 1 h at room temperature. This was followed by drying at room temperature for 24 h, and oven-drying at 100 oC for 2 h.
Seed CoatOH + NaOH → Seed CoatONa + H2O
Chemical compositions
Ash content, moisture content and pH values of the unmodified and mercerized samples were carried out by adopting the standard chemical method of AOAC [12]. Iodine absorption number was determined. 2 g of the sample was weighed into a glass vial containing 250 ml of standard iodine solution and stoppered immediately. The iodine filler mix were shaken vigorously for 1 min, centrifuged at 1600 rpm for 5 min and the iodine solution was decanted completely in one smooth motion into a 50 ml beaker. 20 ml of the decanted solution was pipetted into 250 ml Erlenmeyer flask and filtered with standard solution of sodium thiosulphate (Na2S2O3) until a pale yellow colour was observed. Upon addition 5ml of starch indicator, titration continued until a drop of sodium thiosulphate changed blue colour to colourless. The volume of Na2S2O3 was recorded as volume S while the volume of Na2S2O3 solution, which was titrated against 20ml black sample to the neutral point, was recorded as volume B.
![]()
where S = ml of Na2S2O3 required for the sample, B = ml of Na2S2O3 required for the blank; V = volume of iodine solution used; N = molarity of sodium thiosulphate.
Fourier-Transform InfraRed spectroscopy
Fourier Transform Infra-red (FTIR) spectra of the sample were obtained with a Nicolet AVATAR 360 Fourier Infra-red spectrometer using KBR disks. 1 mg of the sample was dispersed in a matrix of KBR (100 mg) and pressed to form packet. The spectral was measured at a resolution of 4 cm-1 and 32 scans were recorded per sample.
X-ray diffractometry
The X-ray diffraction studies were carried out using a Siemens D5000 X-ray powder diffractometer (20 oC geometry, USA). The fine samples were filled into a sample holder and packed as a density as possible. Then, the sample was mounted into an X-ray diffractometer and copper ka, 2 λ (λ =1.540 µm and 1.544 A; 35 mA) will be generated to determine X-ray pattern. The scan was made from a distraction angle (2) of 1.5 to 70 at a 0.05 step size with a count time of 35. From the resulting X-ray pattern, peak positions were identified using the instruments software and these positions were used to determine the crystalline nature of the filler samples [13].
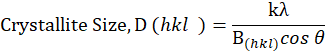
where, k = Scherer constant (0.84), λ = 1.54 µm, B(hkl) = FWHM (Full Width Half Maximum) and θ = Braggs angle corresponding to FWHM
Scanning Electron Microscopy (SEM)
The samples were sprinkled onto the aluminum specimen stubs with double-sided adhesive tape and coated with a 30 nm layer of gold using a sputter coater [Polaron (Fisons) SC 515 VG Microtech, Sussex, UK]. The coated samples were observed using a Scanning Electron Microscope (FESEM Leo Supra 50 VP, Carl-Zeiss SMT, Oberkochen, Germany). Images were captured at different magnifications for morphological studies.
Results and Discussion
Chemical compositions
The unmodified sample exhibits pH of 6.8, which indicates that the sample is less acidic (Table 1).
Table 1. Chemical compositions of unmodified and mercerized pulverized jack bean seed coats
|
Sample |
pH |
Moisture Content (%) |
Ash Content (%) |
Iodine Absorption Number |
|
Unmodified |
6.48±0.10 |
9.50±0.02 |
20.49±0.10 |
1.98±0.01 |
|
Mercerized |
10.97±0.10 |
4.76±0.01 |
18.18±0.10 |
1.00±0.01 |
The mercerized sample is more alkaline than the corresponding unmodified sample. The moisture content of the unmodified sample (9.50%) is higher than the NaOH-treated (mercerized) sample (4.76%). This observation shows that alkali-treatment lowers the moisture absorption by jack bean seed coat fiber. This can be attributed to the presence of a large number of free hydroxyl groups on the hemicelluloses chain, which is polar and hydrophilic [14]. Moisture absorption of natural fiber is a very important parameter for fiber-reinforced composites. During the fiber-reinforcement of composites, the fiber with high moisture content exhibits an inferior interfacial compatibility with the hydrophobic matrix, leading to poor mechanical properties of the fiber reinforced composites [4, 14]. This, in addition to creating micro-cracks on the fiber-reinforced composites, it also weakens the mechanical properties of composites [6, 14]. The moisture content of fiber affected the dimensional stability, electrical resistivity, tensile strength, porosity, and swelling behavior of natural fiber-reinforced composites [15]. Thus, the mercerized sample studied can be used in fabricating fiber-reinforced asbestos, roofing boards, etc.
The ash content determines the availability of minerals in the sample. From the result obtained, the ash content of the unmodified sample is 20.09% while the mercerized sample is 18.18 %. These values suggest that the unmodified sample possesses lower resistance to ageing through oxidation and radiation than the modified.
The iodine absorption number is used to confirm the reinforcing ability of the sample. The mercerized derivative of pulverized jack bean seed coats exhibits approximately twice the iodine absorption number of the unmodified. This is a measure of degree of absorption surface of fibers. Sodium ion (Na+) is capable of expanding the lattice plane of the granules [8], thereby increasing the surface area, especially for absorption of water molecules, although weak absorption on the surface may be experienced by other molecules.
FTIR spectroscopy
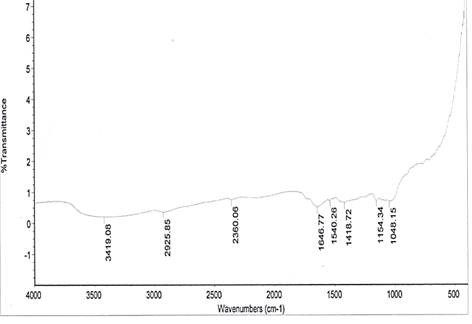
The spectral characteristics of the unmodified and NaOH-treated (mercerized) pulverized jack bean seed coats are presented in Fig. 1. Absorption bands 1048 cm-1 and 1097.33 cm-1 for the respective unmodified and mercerized samples are close to the absorption band of 1057 cm-1, which has been attributed to the C-O stretching modes of the hydroxyl and ether groups in the cellulose [16].
Very strong NO of nitro group is observed at 1540.26 cm-1, which disappears upon modification. This may account for the differences in the pH values of the samples. Nitro groups have high dipole moments, that is, greater polarity, resulting in low solubility in water. Hence, the disappearance of this group in the modified derivative of the sample may favour the relative solubilization of the mercerized pulverized jack bean seed coat in water. The reports of other researchers for coconut fibers [17] and coir fibers [18] have shown the disappearance of absorption bands in NaOH-treated.
a)
 b)
b) 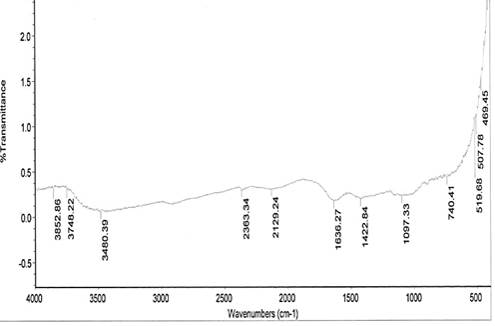
Figure 1. FTIR spectra of: (a) unmodified and (b) mercerized pulverized jack bean seed coat
From the spectra, there are 3748.22 and 3852.86 cm-1 absorption bands observed for the mercerized sample, indicating free hydroxyl group (OH) stretch from the cellulose and lignin unit of the fiber.
The absorption bands of 3419.08 cm-1 and 3480.00 cm-1 observed for unmodified and mercerized samples respectively, are indications of (OH) stretch of normal polymeric hydroxyl group from the cellulosic and lignin units of the fiber. A broad peak of 3418 cm-1 signal could be cellulose hydroxyl, which is a characteristic signal of lignocellulosics [19].
From the spectra, the absorption band 2129.24 cm-1 indicates C≡C terminal alkynes (mono substituted). This makes mercerized samples more reactive than the unmodified. The higher the degree of unsaturation, the higher the reactivity of a substance.
X-ray patterns
Table 2 shows the characteristics of three major X-ray diffraction peaks of the unmodified and mercerized pulverized jack bean seed coats. X-ray diffraction peaks for the unmodified sample appear at 24.09o, 18.31o and 47.57o 2θ, corresponding to interplanar d-spacing of 3.69Å, 4.84 Å and 1.19 Å. Likewise, the mercerized sample exhibits X-ray diffraction peaks, which appear at 24.06o, 18.30o and 47.53o 2θ, corresponding to interplanar d-spacing of 3.670Å, 4.84 Å and 1.91 Å. The modification process does not significantly alter the diffraction peaks and patterns of the unmodified samples. These peaks are indicative of mixes of A- and B- polymorphs. Hence, both the unmodified and mercerized samples are of C-type pattern.
Table 2. Major peak characteristics of unmodified and mercerized samples
|
Sample |
Peak I |
Peak II |
Peak III |
|||||||||
|
I |
2θ |
d |
RI |
I |
2θ |
d |
RI |
I |
2θ |
d |
RI |
|
|
Unmodified |
1954 |
24.09 |
3.69 |
100 |
574 |
18.31 |
4.84 |
27 |
328 |
47.57 |
1.19 |
22 |
|
Mercerized |
2201 |
24.06 |
3.70 |
100 |
543 |
18.30 |
4.84 |
25 |
276 |
47.53 |
1.91 |
13 |
|
I = Intensity (counts); 2θ = Braggs angle; d = d-spacing; RI = relative intensity |
||||||||||||
Table 3 depicts the crystallite sizes of both the unmodified and mercerized derivatives of pulverized jack bean seed coats. The unmodified sample exhibits 8.60 as crystallite size while the mercerized is 10.10. This is an evidence of the effect of decreased moisture content upon alkali treatment of the fiber, which would, without doubt lead to improvement in the mechanical strength, stress-strain profiles of the mercerized fiber studied. This is in league with the observations reported for luffa sponge fibers [14].
Table 3. Major peak characteristics of unmodified and mercerized samples
|
Sample |
B(hkl) |
θ (2θ0) |
Crystallite Size, D(hkl) |
|
Unmodified |
0.17 |
24.09 |
8.60 |
|
Mercerized |
0.14 |
24.06 |
10.10 |
|
(hkl)= FWHM (Full Width Half Maximum); θ(corresponding Braggs angle to FWHM); |
|||
Scanning Electron Microscopy
The particle properties of unmodified and mercerized samples with the aid of SEM (Scanning Electron Microscope) are shown in Table 4. From the table, the unmodified sample exhibits higher particle properties in terms of circle equivalent diameter, major axis, minor axis, circumference, convex hull, circumscribed circle diameter, area, volume by area and pixel count. This is an evidence of reduced moisture absorption and improved compatibility of the fiber with polymer matric in fabrication upon alkali treatment.
Table 4. Particle properties of unmodified and mercerized pulverized jack bean seed coats
|
Property |
Sample |
|
|
Unmodified |
Mercerized |
|
|
Circle equivalent diameter (µm) |
90.20 |
78.30 |
|
Major axis (µm) |
118.00 |
96.60 |
|
Minor axis (µm) |
70.00 |
64.50 |
|
Circumference (µm) |
518.00 |
363.00 |
|
Convex hull (µm) |
375.00 |
293.00 |
|
Circumscribed circle diameter (µm) |
147.00 |
114.00 |
|
Area (µm²) |
7.34 × 103 |
5.49 × 103 |
|
Volume by area (µm³) |
5.46 × 105 |
3.51 × 105 |
|
Pixel count |
9309.00 |
7201.00 |
|
Elongation |
0.375 |
0.328 |
The micrographs of the unmodified and mercerized samples observed with scanning electron microscope are displayed in Fig. 2.
(a)
 (b)
(b)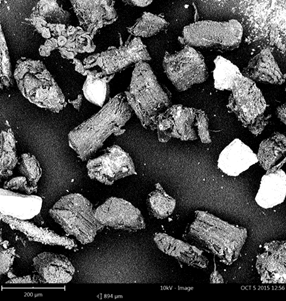
Figure 2. Scanning electron micrographs of: (a) unmodified and (b) mercerized samples
The granules of the unmodified sample do not assume a conventional shape. Hence, they are irregular, non-uniform granules. The granules of the mercerized sample are cylindrical and rod-like shapes. The surface outlook of the mercerized sample appears more crystalline and fibrous than the unmodified. This, among other things, may account for the high crystallinity index and its possible compatibility with other polymer matrices in industrial fabrications.
Conclusions
The pulverized jack bean seed coat has been studied with successful modification through mercerization as revealed by the FTIR spectral profiles. Absorption bands resembling the characteristic signal of lignocellulosics is observed in the mercerized derivative of the pulverized jack bean seed coat. The alkali treated (mercerized) sample tends to be more reactive than the unmodified due to the unsaturated alkyne monosubstituted group (C≡C) observed after modification. The XRD profiles show that the crystallite size is more favoured by modification. Both the unmodified and the modified derivative exhibit the same XRD pattern of C-type, which is a mix of A- and B- polymorphs. The modified pulverized jack bean seed coat is weakly acidic with comparatively low moisture content, high resistance to oxidative degradation and high surface area compared to its unmodified form. This tends to give improved mechanical properties of the treated fiber. The SEM analysis shows irregular shapes of the unmodified granules and mixes of cylindrical and rod-like shapes for the mercerized granules. The mercerized fiber possesses higher tendency of application in the fabrication of fiber-polymer composites than the unmodified fiber. The treated fiber studied is cheap, accessible and environmental friendly compared to other synthetic fibers.
References
1. Shirikant N., Lascala J. J., Can E., Morye S. S., Williams G. I., Palmese G. R., Development and Application of Triglyceride-Based Polymers and Composites, Journal of Applied Polymer Science, 2001, 82, p. 703-723.
2. Dweib M. A., Hu B., ODonnell A., Shenton H. W., Woo R. P., All Natural Composite Sandwich Beams for Structural Applications, Composite Structures, 2004, 63, p. 147-157.
3. Adekunle K. F., Surface Treatments of Natural FibresA Review: Part 1, Open Journal of Polymer Chemistry, 2015, 5, p. 41-46.
4. Sgriccia N., Hawley M., Misra M., Characterization of natural fiber surfaces and natural fiber composites, Composites Part A: Applied Science and Manufacturing, 2008, 39(10), p. 1632-1637.
5. Rosa I. M. D., Kenny J. M., Maniruzzaman M., Moniruzzaman M., Monti M., Puglia D., Santulli C., Sarasini F., Effect of chemical treatments on the mechanical and thermal behaviour of okra (abelmoschus esculentus) fibres, Composite Science and Technology, 2011, 71(2), p. 246-254.
6. Ku H., Wang H., Pattarachaiyakoop N., Trada M., A review on the tensile properties of natural fiber reinforced polymer composites, Composites Part B: Engineering, 2011, 42(4), p. 856-873.
7. Hashim M. Y., Roslan M. N., Amin A. M., Mujahid A., Zaidi A., Ariffin S., Mercerization Treatment Parameter Effect on Natural Fiber Reinforced Polymer Matrix Composite: A Brief Review, International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering, 2012, 6(8), p. 778-784.
8. Liu F. P., Wolcott M. P., Gardner D. J., Rials T. G., Characterization of the Interface between Cellulosic Fibers and a Thermoplastic Matrix, Composite Interfaces, 1994, 2, p. 419-432.
9. Akpapunam M. A., Sefa-Dedu S., Some physical properties and antinutritional factors of raw, cooked and germinated jack bean (Canavalia ensiformis), Food Chemistry, 1997, 59(1), p. 121-125.
10. Oladebeye A. O., Oshodi A. A., Amoo I. A., Karim A. A., Hydroxypropyl derivatives of legume starches: Functional, rheological and thermal properties, Starch/Stärke, 2013, 65, p. 762-772.
11. Oladebeye A. O., Physicochemical characterization of native, modified and nano starches of selected tubers and seeds, PhD Research Dissertation, Department of Chemistry, Federal University of Technology, Akure, Nigeria, 2014.
12. AOAC, Association of Official Analytical Chemists, Official Methods of Analysis (8th Ed.). Washington D.C., Association of Official Analytical Chemists, 1995.
13. Jayakody L., Hoover R., Liu Q., Donner, E., Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea spp) starches grown in Sri Lanka, Carbohydrate Polymers, 2007, 69, p. 148-163.
14. Chen Y., Su N., Zhang K., Zhu S., Zhu Z., Qin W., Yang Y., Shi Y., Fan S., Wang Z., Guo Y., Effect of fiber surface treatment on structure, moisture absorption and mechanical properties of luffa sponge fiber bundles, Industrial Crops and Products, 2018, 123, p. 341-352.
15. Yusriah L., Sapuan S. M., Zainudin E. S., Mariatti, M., Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fiber, Journal of Cleaner Production, 2014, 72, p. 174-180.
16. Paiva M. C., Ammar I., Campos A. R., Cheikh R. B., Cunha A. M., Alfa fibres: mechanical, morphological and interfacial characterization, Composite Science and Technology, 2007, 67, p. 1132-1138.
17. Brígida A. I. S., Calado V. M. A., Gonçalves L. R. B., Coelho M. A. Z., Effect of chemical treatments on properties of green coconut fiber, Carbohydrate Polymers, 2010, 79, p. 832-838.
18. Rout J., Tripathy S. S., Nayak S. K., Misra M., Mohanty A. K., Scanning electron microscopy study of chemically modified coir fibers, Journal of Applied Polymer Science, 2001, 79, p. 1169-1177.
19. Abdul-Khalil H. P. S., Khairul A., Bakare I. O., Bhat, I., Thermal, spectroscopic and flexural properties of anhydride modified cultivated Acacia spp, Wood Science and Technology, 2011, 45, p. 547-606.