Synthesis, characterization and computational studies on the corrosion inhibitive potentials of (e)-3-(2-p-tolyldiazenyl)-1-nitrosonaphthalen-2-ol
Solayide Justinah AMOKO1,3, Olawale Folorunso AKINYELE2*, Oluwatoba Emmanuel OYENEYIN1, Dare Samson OLAYANJU2, Christopher Olu ABOLUWOYE1
1Department of Chemical Sciences, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
2Department of Chemistry, Obafemi Awolowo University, Ile-Ife, Nigeria
3Department of Chemistry, Adeyemi College of Education, Ondo, Ondo State, Nigeria
Emails: 1temiamoko@gmail.com, 2ofakinyele@oauife.edu.ng, 3emmanueltoba@gmail.com, 4oladare78@yahoo.com and 5oluaboluwoye@yahoo.com.
*Corresponding Author Phone: +2348023410301
Abstract
A novel (E)-3-(2-P-Tolyldiazenyl)-1-nitrosonaphthalen-2-ol (NAD) has been synthesized and characterized via Fourier Transform Infrared (FTIR), UV/Vis, 1H-NMR and 13C-NMR spectroscopic techniques. Weight loss analysis showed that this molecule possesses excellent corrosion inhibitive potentials. Variations of inhibition efficiency with concentration and temperature were observed and the results obtained show that inhibition efficiency increases with increase in concentration while it reduces with an increase in temperature. The efficiency of this molecule was validated with theoretical models using the density functional theory (DFT) method with the hybrid functional, Becke Three Lee Yang Parr (B3LYP) correlation with 6-31G* basis set. It was observed that the molecule has very good corrosion inhibitive potentials.
Keywords
Density Functional Theory (DFT); Polarization; Activation parameters; Azo-dye; Thermodynamic parameter; Adsorption; Inhibition efficiency
Introduction
One of the most practical ways to prevent corrosion of metals and alloys in acidic media, a scourge peculiar to the oil and gas industries, is to use corrosion inhibitors, which are mainly organic compounds containing heteroatoms like sulphur, nitrogen, oxygen and phosphorus with lone pair of electrons and as a result facilitate adsorption on metal surfaces [1-4]. These inhibitors are added with the aim of reducing metal loss when in contact with acid media. Azo dyes possess π-electrons systems and heteroatoms in the moieties, this enhances adsorption on mild steel through the formation of covalent bonds [5,6]. They are adsorbed on the metal surface by forming protective layers that prevent attacks by acid media used in pickling, descaling and oil well acidizing. Methods such as the gravimetric; which gives information about the average rate of corrosion and inhibition efficiency [6-8], potentiodynamic polarization [8,9], electrochemical impedance spectroscopy [10], Fourier transform infra-red (FTIR) spectroscopy [6] and scanning electron microscope (SEM) [6,8] have been used for corrosion inhibition studies. Recently, quantum chemical calculations have been employed in investigating and predicting the corrosion inhibition potentials of molecules [4,6,10,11]. This is because they can be used to explain physical parameters like reactivities and electronic behaviours. Since it is important to describe the electronic behaviours, the density functional theory (DFT) is usually used in predicting and understanding chemical processes like corrosion inhibition processes either as a method to validate experimental findings or solely to explain inhibitive potentials of organic molecules [10-18]. Azo dyes have been extensively investigated and reported as corrosion inhibitors [8,9,12]. This may be due to the ability of complex formation between metal-ions and the azo nitrogen binding at the electrode surface [8], steric effects and electron density of donor atoms [13]. This work presents the validation of the findings from weight loss and potentiodynamic polarization experiments.
Preparation of Mild Steel
Commercially available mild steel was bought and mechanically machined into dimension 3 cm × 3 cm × 0.05 cm with hole at the centre for easy hooking. Each were polished with evenly gritted emery paper; washed with distilled water and degreased with acetone by dipping them in acetone for five minutes and dried over CaCl2 in desiccators overnight [19].
Synthesis of NAD
NAD was synthesized using the method reported in literature (Scheme 1) [20]. A suspension of P-Toluidine (2.8 g, 40 mmol) in hydrochloric acid (36 ml) and water (16 ml) was heated to around 70oC until complete dissolution. The clear solution poured into ice was diazotized below 5oC with sodium nitrite (2.8 g, 40 mmol) dissolved in water (10 ml). The resulting cold diazonium solution was then added slowly with continuous vigorous stirring over the course of 45 mins at 0oC to a solution of 1-nitrosol-2-naphatol (6.92 g, 40 mmol) in water (75 ml) containing sodium hydroxide (1.6 g) and sodium carbonate (14.8 g). The product was collected by filtration and washed with water then later recrystallized from ethanol three times.
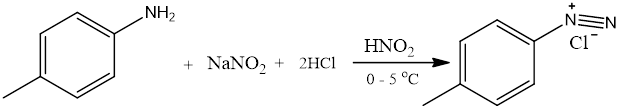
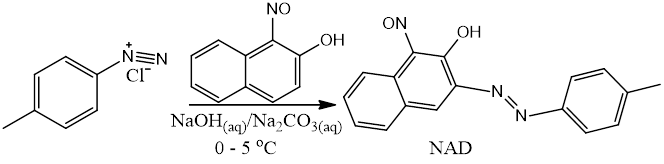
Scheme 1. Synthetic route of NAD
The synthesized brownish-yellow molecule has a chemical formula of C17H16N3O2, molecular weight of 294.334, melting point 110-112 oC and 98% yield. The spectra are shown in Figures 1, 2, 3 and 4.
FTIR (KBr, cm-1): 3443 (O-H), 3066 (aromatic C-H), 1620 (aromatic C=C), 1406 (N=Nstr), 1070 (C-O)
UV/Vis λmax (nm): 262 (π-π*), 399 (Inter-molecular charge transfer, IMCT) and 415 (n-π*)
1H-NMR (DMSO-d6) δ: 8.89 (s, 1H, Hg), 7.75 (d, 2H, He), 7.62 (d, 2H, Hd), 7.55 (m, 3H, Hc), 6.42 (d, 2H, Hb), 2.50 (s, 3H, Ha)
13C-NMR (DMSO-d6) δ: 145 (s), 130 (d), 129 (m), 127 (s)
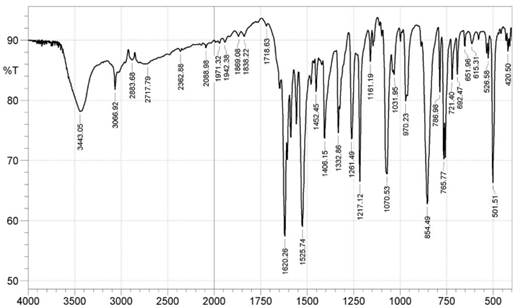
Figure 1. Infrared spectrum of NAD
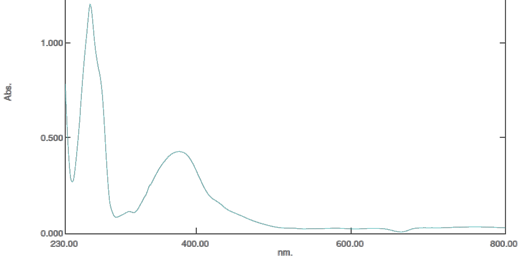
Figure 2. The UV spectrum of NAD
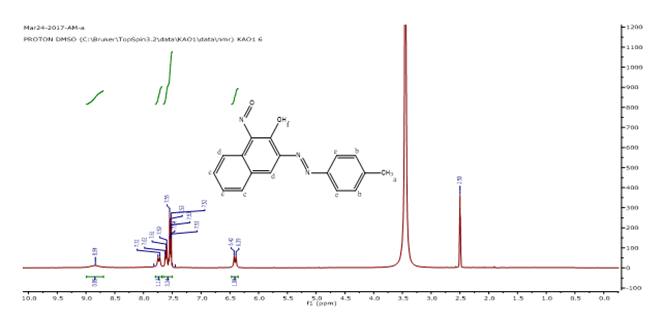
Figure 3. 1HNMR spectrum of NAD
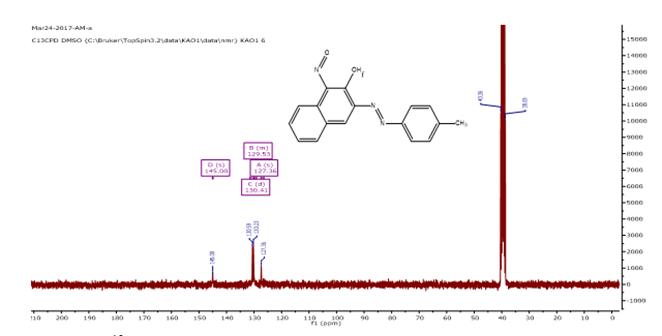
Figure 4. 13CNMR spectrum of NAD
Preparation of solution
0.5 M HCl was prepared using hydrochloric acid of standard grade and distilled water which serve as the corrodent. The inhibitor (NAD) was added to this test solution in a dosage of 10 – 50 ppm. Steel coupons for the gravimetric experiment were introduced into beakers containing this tested solution and the tests were performed in thermostated water bath at each test temperature (25, 35, 45, 55 and 65oC) for 6 hrs.
Weight loss experiment
The
pre-treated Mild steel coupons weighed with the aid of analytical balance
(0.0001 sensitivity) were immersed in 100 ml 0.5 M HCl test solution loaded
with different concentrations of the inhibitor (10 – 50 ppm) in an open beaker
for 6 hours at varying temperature (298 – 338K at 10 K interval)
using thermostated water bath. Samples were withdrawn from the test solution
after 6 hrs, brushed with brittle brush, washed with distilled water, rinsed in
acetone and dried in oven for 15 mins at 40 oC before reweighing
[19]. Each of this experiment was performed in triplicate for accurate results
and the mean of the final weight was used in the calculation. From the weight
loss results, the corrosion rate, CR, degree of surface coverage,
θ and the percentage inhibition efficiency, IE % were determined using
Equations 1, 2 and 3 respectively.
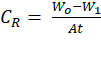 1
1
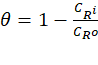 2
2
![]() 3
3
where Wo and W1 are the weight loss (g) of mild steel in the absence and presence of the inhibitor respectively. A is the area of the mild steel (cm2), t is the immersion time (hrs). CRi and CRo are the corrosion rates (g cm-2 hr-1) in the presence and absence of the inhibitor respectively.
The adsorption of an inhibitor on metal surface is in form of a
substitution reaction, with the inhibitor substituting the water molecules
adsorbed on the metal surface and in order to describe this reaction,
adsorption isotherms are usually employed. The Langmuir adsorption isotherm
(Equation 4) was employed in this study to explain the adsorption of NAD on the
mild steel [22, 23].
![]() 4
4
where C is the concentration of inhibitor, Kads the equilibrium adsorption constant and θ the extent of surface coverage.
To further explain the adsorption process, thermodynamic parameters were
calculated. A relation (equation 5) was used to calculate the standard Gibb’s
free energies of adsorption (∆Goads) from the
various values of adsorptive equilibrium constant at specific temperature (Kads)
[25].
![]() 5
5
where R is
universal gas constant, ∆Gads is the free energy of adsorption
and 55.5 is the molar concentration of water in the solution known as the
isocratic contribution. A plot of ∆Goads
against temperature using Equation 6 gave the enthalpy, ∆Ho
and entropy ∆So of adsorption as intercept and slope respectively
[26].
![]() 6
6
The adsorption behavior of NAD was further explained using the kinetic
model. The apparent activation energies, Ea, for the corrosion
process and the pre-exponential factors, A, were determined using the plot of
lnCR against 1/T (Figure 9) as given by the Arrhenius equation 7
[28]. Values obtained are given in Table 2.
![]() 7a
7a
![]() 7b
7b
Using the transition state Equation 8, values of enthalpy, ΔH* and
entropy, ΔS* of activation were calculated using the slope and intercepts
of the plots of ln(CR/T) against 1/T (Figure 6.0) and these are
given in Table 3.
![]() 8
8
where h is the Planks’ constant, N the Avogadro’s number, T the absolute temperature and R the universal gas constant.
Potentio-dynamic polarization experiments
Electrochemical experiments were conducted in a three-electrode cell using corrosion Lab and Versa stat 4 software under standard condition. A platinum sheet was used as the counter electrode (CE), saturated calomel electrode (SCE) as the reference electrode, while the mild steel machined into rectangular specimens with an exposed area of 1 cm2 as the working electrode (WE). All potentials were measured against the SCE (0.2412 V with respect to the normal hydrogen electrode). Measurements were carried out in naturally aerated and unstirred solutions at 298 K using a thermostated water bath. The open current potential (OCP) was established by immersing the working electrode in the test solution for 10 mins while the potentiodynamic polarization studies were carried out by sweeping the applied potential from -250 mV to +250 mV at OCP with a scan rate of 1mVs-1 while the current, I, is recorded.
Equation 9 helped to give the inhibition efficiency calculated from the
values of corrosion current density extrapolated from the tafel curves [29].
![]() 9
9
where, ![]() and Icorr are the corrosion
densities in the absence and presence of the inhibitor respectively.
and Icorr are the corrosion
densities in the absence and presence of the inhibitor respectively.
Computational studies
Quantum chemical calculations were performed on the most stable conformer of NAD after a conformation search has been carried out using the molecular mechanic force field (MMFF) method. The geometry of the molecule was optimized using the DFT with B3LYP correlation and a high basis set, 6-31G* using computational chemistry software. The energies of the frontier molecular orbitals, FMOs (HOMO and LUMO) were obtained, it affords us to understand the sites that are prone to nucleophilic and electrophilic attack and also used to determine the energy band gap, Eg (ELUMO - EHOMO) [11,12,14]. Other properties like the global reactivity descriptors like the chemical hardness (η), softness (δ), electronegativity (χ) and global electrophilicity index (ω) were calculated from the FMOs. The dipole moment (μ) and the fraction of electron transferred (ΔN) were also calculated, all in gas phase.
The chemical hardness, a direct consequence of the energy band gap was
calculated using Equation 10 Its
value is 1.56 eV [11,12,14];
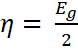 10
10
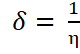 11
11
Other global reactivity descriptors calculated are the electronegativity
in Equation 12
[11,12,14]. NAD has 4.53 eV as its electronegativity, a value lower
than that of iron as expected.
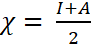 12
12
where; I = -EHOMO and
A = -ELUMO and the global electrophilicity index in Equation 13
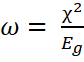 13
13
A good inhibitor is characterized by donation of electrons from its HOMO orbitals to the metal’s d orbitals and also through the acceptance of electrons from the metal’s d orbitals to its orbitals.
Fraction of electron transferred was calculated using Equation 14 [11,12].
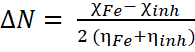 14
14
where χFe and χInh are the electronegativities of iron (representing bulk metal) and the inhibitor molecule respectively. ηFe and ηInh are the absolute hardness of iron and the inhibitor molecule respectively. χFe has a theoretical value of 7 eV while that of ηFe is 0 eV [11,14].
Results and discussion
Gravimetric analysis
The results obtained showed that at different concentrations of NAD (0.00005, 0.0001, 0.00015 and 0.0002) mol/L, the % inhibition efficiency increases as the concentration of the inhibitor increases at a fixed temperature but reduces as the temperature is increased i.e the corrosion rate reduces with an increase in concentration at a fixed temperature and increases with an increase in temperature (Figures 5 and 6). This is in line with what researchers observed in their work [4,6,16].
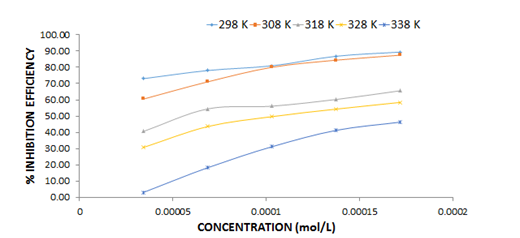
Figure 5. Variation of inhibition efficiency with concentration of NAD
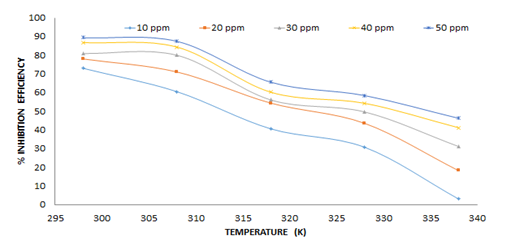
Figure 6. Variation of inhibition efficiency of NAD with temperature
Adsorption considerations
The plot of C/θ against C at different temperatures (298 –
338 K) gave the parameters listed in Table 1 while Figure 7 showed these plots.
Both linear correlation coefficients (R2) and slope values are
closer to 1, suggesting that the adsorption obeys Langmuir adsorption isotherm.
The little deviation from unity observed in the slope may be traced to the
repulsion force in the adsorption layer [24].
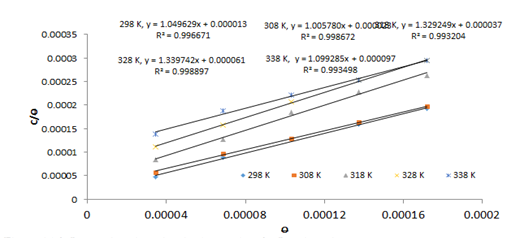
Figure 7. Langmuir adsorption isotherm plots for NAD adsorption
The decrease in the adsorptive equilibrium constant (Kads) values with temperature gave an indication that at relatively lower temperatures, strong adsorption of NAD molecules onto the mild steel surface occurred. On the other hand, already adsorbed molecules of NAD got desorbed from the mild steel surface as experimental temperature increases [23].
From the plot (Figure 8), -41.861 kJ mol-1 and -0.0136 kJ mol-1K-1 were obtained for ∆Hoads and ∆Soads, respectively (Table 1).
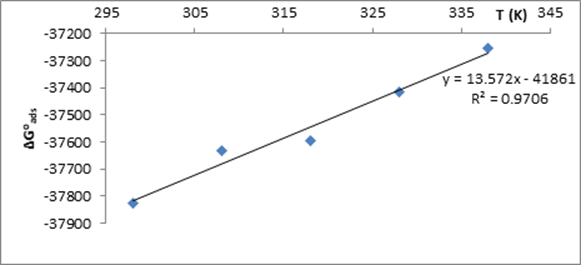
Figure 8. Determination of
the Enthalpy, ∆Hoads and Entropy, ∆Soads,
of Adsorption of NAD on the Mild Steel Surface in 0.5 M HCl
Table 1. Values from Langmuir adsorption isotherm and thermodynamics study
|
Compd |
Temp. (K) |
R2 |
Slope |
1/K (mol/l) |
K (l/mol) |
∆G (kJ/mol) |
∆S (kJ/mol K) |
∆H (kJ/mol) |
|
NAD |
298 |
0.996671 |
1.049629 |
0.000013 |
76923.08 |
-37.8249 |
-0.01357 |
-41.861 |
|
308 |
0.998672 |
1.00578 |
0.000023 |
43478.26 |
-37.6332 |
|
|
|
|
318 |
0.993204 |
1.329249 |
0.000037 |
27027.03 |
-37.5982 |
|
|
|
|
328 |
0.998897 |
1.339742 |
0.000061 |
16393.44 |
-37.4171 |
|
|
|
|
338 |
0.993498 |
1.099285 |
0.000097 |
10309.28 |
-37.2544 |
|
|
∆Goads were obtained as negative owing to spontaneity of adsorption. In general, value of ∆Goads around -20 kJmol-1 indicates physical adsorption and value of ∆Goads of -40 kJ/mol and above is an indication of chemisorption [27]. ∆Goads obtained in this study were around -37 kJ/mol which gave a hint of complex adsorption mechanism. The negative value of ∆Soads showed the decrease in the degree of translational freedom of the molecules as it is being adsorbed on the mild steel surface. Negative value of ∆Hoads obtained indicated the exothermic nature of the adsorption process [26]. To differentiate physisorption from chemisorptions in an exothermic adsorption process, ∆Hoads around -40 kJmol-1 is an indication of physisorption while, ∆Hoads tending to -100 kJmol-1 indicates chemical adsorption of the inhibitor molecules. -41.801 kJ/mol obtained for ∆Hoads can be assigned to physical adsorption of NAD molecules on the mild steel surface.
Kinetic studies and activation parameters
The results of the kinetic studies and activation parameters are given in Figure 9 and Table 2.
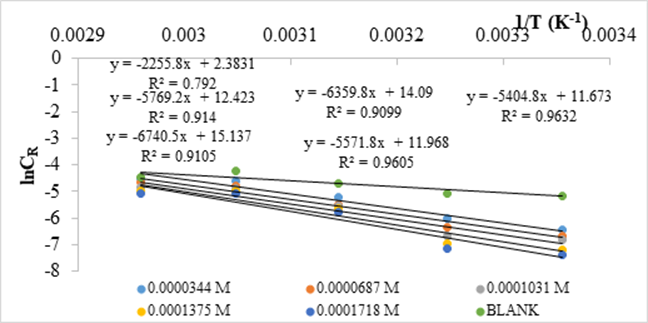
Figure 9. Apparent activation energy determination for the corrosion process in 0.5 M HCl in the absence and presence of NAD
Values of apparent activation energies increase in the presence of NAD than that of pure acidic medium [28]. Higher energies are required for the corrosion reaction as the concentration of NAD increases in the inhibited solution. The higher values of Ea also indicate the physical adsorption that occurred in the first stage [29,28]. The pre-exponential factor, A, in the Arrhenius equation is related to the number of active centers [28]. The larger values of A in the presence of NAD compared to that of the uninhibited solution (Table 3) implied that most of the active sites are blocked by the adsorption of the inhibitor [28].
Table 2. Activation energy, Ea and pre-exponential factor A for the corrosion process in 0.5 M HCl
|
Compd |
Concentration (ppm) |
Slope |
Ea (J/mol) |
lnA |
A |
|
NAD |
BLANK |
-2255.8 |
18754.72 |
2.3831 |
10.83845 |
|
0.0000344 |
-5404.8 |
44935.51 |
11.673 |
117359.8 |
|
|
0.0000687 |
-5571.8 |
46323.95 |
11.968 |
157629.1 |
|
|
0.0001031 |
-5769.2 |
47965.13 |
12.423 |
248450.8 |
|
|
0.0001375 |
-6359.8 |
52875.38 |
14.09 |
1315859 |
|
|
0.0001718 |
-6740.5 |
56040.52 |
15.137 |
3749001 |
Analysis of data in Table 3 revealed an increase in ΔH* and ΔS* values in the presence of NAD an indication of the increase in energy barrier of corrosion process in the presence of NAD [28] which agreed with the values of activation energy, Ea, presented in Table 4. Positive values of enthalpies of activation ΔH* in the absence and presence of NAD suggested an endothermic nature of the mild steel dissolution [30–33]. Negative values obtained for ΔS* in the absence and presence of NAD suggested the formation of the activated complex in the rate determining step to be associative rather than been dissociative and hence, there was decrease in disorderliness as the reaction moves from the reactants to the activated complex [33,34].
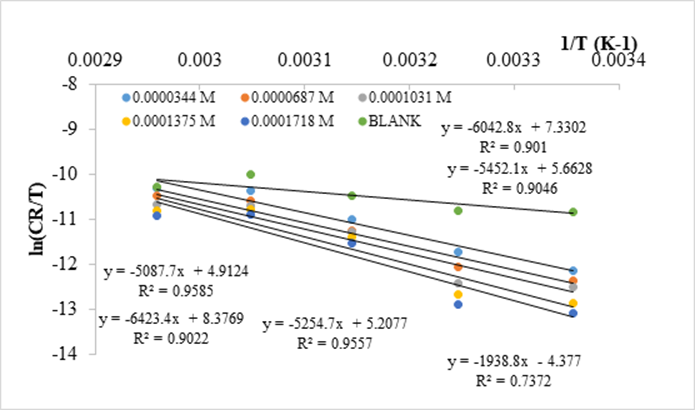
Figure 10. Transition state determination of enthalpy and entropy of activation in 0.5 M HCl in the absence and presence of NAD
Table 3. Values for the corrosion activation parameters of mild steel in 0.5 M HCl in the absence and presence of different concentration of NAD
|
Compd |
Conc. (m) |
Slope |
Intercept |
Enthalpy (J/mol) |
Entropy (Jmol-1k-1) |
|
NAD |
BLANK |
-1939 |
-4.3770 |
16120.85 |
-233.93 |
|
0.0000344 |
-5088 |
0.9124 |
42301.63 |
-189.954 |
|
|
0.0000687 |
-5255 |
5.2077 |
43690.07 |
-154.243 |
|
|
0.0001031 |
-5452 |
5.6628 |
45327.93 |
-150.459 |
|
|
0.0001375 |
-6043 |
7.3302 |
50241.50 |
-136.597 |
|
|
0.0001718 |
-6423 |
8.3769 |
53400.82 |
-127.894 |
Potentiodynamic polarization
The polarization curves of the mild steel in 0.5 M HCl solution in the absence and presence of different concentrations of NAD are given by Figure 11. Other parameters such as the corrosion potential (Ecorr), corrosion current density (Icorr), anodic Tafel slope (βa), cathodic Tafel slope (βc) and percentage inhibition efficiency (% IE) are summarized in Table 4.
Generally, organic inhibitors can be classified as cathodic, anodic or mixed-type inhibitor depending on whether there is decrease in the corrosion current densities at the cathodic or anodic or both sides, respectively, of the Tafel curve on addition of the inhibitor [36, 35]. It was observed from Figure 11 that both the cathodic and the anodic corrosion current densities decreased considerably most especially in the presence of 30 ppm NAD indicating that NAD is a mixed type inhibitor.
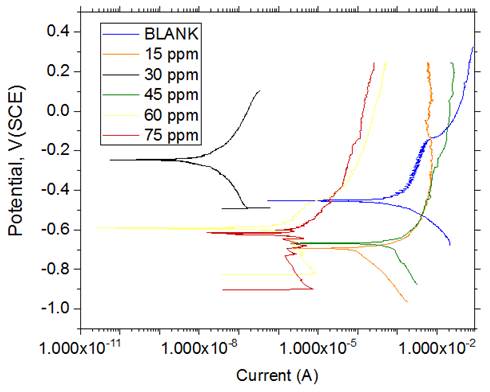
Figure 11. Potentiodynamic polarisation curve for mild steel in 0.5 M HCl solution in the absence and presence of NAD
Table 4. Polarization parameters and inhibition efficiencies for mild steel in 0.5 M HCl solution in the absence and presence of different concentrations of NAD at 298 K
|
Compd |
Conc (g) |
- βc (mV) |
βa (mVdec-1) |
CR mpy |
-Ecorr (mV) |
Icorr (mA) |
% IE |
|
NAD |
Blank |
111.71 |
292.71 |
461.40 |
451.4 |
1.009000 |
- |
|
0.015 |
248.49 |
55.83 |
76.15 |
703.2 |
0.166000 |
83.55 |
|
|
0.030 |
148.03 |
148.03 |
0.0059 |
248.1 |
0.000012 |
99.99 |
|
|
0.045 |
269.854 |
221.63 |
397.36 |
703.1 |
0.869803 |
13.80 |
|
|
0.060 |
146.87 |
146.87 |
0.4835 |
594.3 |
0.001058 |
99.90 |
|
|
0.075 |
270.91 |
270.91 |
2.253 |
618.4 |
0.004931 |
99.51 |
This suggested that NAD has retarding effect on both cathodic and anodic half reactions. Analysis of data in Table 3.2 equally revealed changes in the values of βa and βc in the presence of NAD indicating the influence of the inhibitor on the anodic and cathodic reactions [37]. The displacement of Ecorr to the more negative values in the presence of NAD suggested the retarding effect of the inhibitor on both sides of the tafel curve. Hence, the corrosion reaction of the mild steel is inhibited. An increase in the inhibition efficiency of NAD in the inhibited solution as compared to the uninhibited solution was also observed suggesting the adsorption of NAD on most of the active sites causing considerable decrease in the corrosion current of the mild steel.
Quantum chemical calculations
The energy band gap is 3.12 eV; this is indicative that there is an inherent electron donating ability and that there is an interaction between the inhibitor and the metal surface with high HOMO energy of -6.09 eV, indicative that the inhibitor has excellent donating ability and a low LUMO energy of -2.97 eV, indicative that the inhibitor can accept electrons from metal’s d orbitals.
Softness is the inverse of hardness and it determines the ability of a molecule to react. Soft molecules are more reactive than hard ones because of the ease with which they could offer electrons to an acceptor [15, 16]. It is calculated according to Equation 11 [11,12,14] and of a value of 0.64 eV-1. The hard and soft acids and bases principle (HSAB) explains that soft acids tend to react with soft bases and hard acids with hard bases. A bulk metal, whose chemical hardness is assumed to be zero very soft, a soft acid, therefore, they tend to react with soft bases (in this case, the inhibitor). This means that the inhibitor must have a high softness value, this enhances high adsorption and hence high corrosion inhibition efficiency [15,16].
As the inhibitor is brought in contact with the bulk metal, electrons flow from the lower χInh to the higher χFe. ΔN represents the inhibition effect resulting from electron donations and inhibition efficiency can be seen as effective if ΔN < 3.6 [15,17]. ΔN for NAD is 0.79, an indication that it has the ability to donate electrons. This has been earlier confirmed by the low hardness and high softness values.
Also, the dipole moment is an important parameter to ascertain if electrons are distributed in the molecular structure and if there is a strong dipole-dipole interaction between NAD and the metal surface. NAD has a dipole moment of 4.49 Debye, an indication that the inhibition absorption is enhanced through electronic force [18]. The quantum chemical parameters are shown in Table 5 while the HOMO and LUMO orbital diagram are displayed in Figures 11b and c.
Table 5. Quantum chemical parameters for NAD
|
Properties |
EHOMO(eV) |
ELUMO(eV) |
Eg(eV) |
η (eV) |
δ(eV-1) |
χ(eV) |
ω(eV) |
μ(D) |
ΔN |
|
NAD |
-6.09 |
-2.97 |
3.12 |
1.56 |
0.64 |
4.53 |
6.58 |
4.49 |
0.79 |
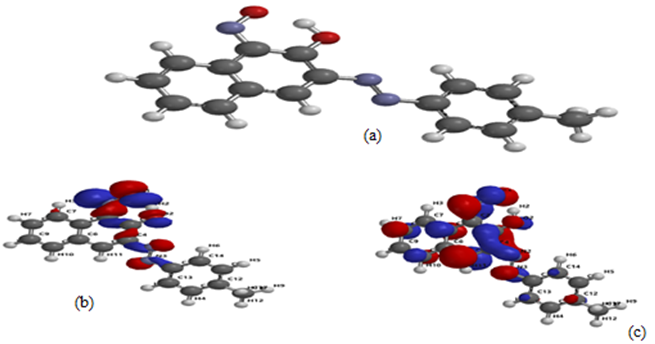
Figure 12. a. Optimized structure, b. Highest Occupied Molecular Orbital (HOMO) and c. the Lowest Unoccupied Molecular Orbital (LUMO) of NAD
Conclusions
The study synthesised and characterised (E)-3-(2-P-Tolyldiazenyl)-1-Nitrosonaphthalen-2-ol with its inhibitory performance evaluated using gravimetric and electrochemical methods. It also described NAD as a good inhibitor for mild steel in acidic environment with Inhibition efficiency increasing with increase in concentration and decreasing with increase in temperature. Electrochemical measurement using potentiodynamic polarization revealing a mixed-type organic inhibitor. Considerable inhibitory efficiencies were obtained in the presence of NAD indicating good agreement with that by weight loss. The adsorption of NAD on mild steel obeys Langmuir adsorption isotherm and is an exothermal and entropy decreasing process while the adsorption mechanism follows physical adsorption. The values of activation parameters (Ea, ∆H* and ∆S*) increased in the presence of NAD. Hence, conclusion could be made that the adsorbed NAD molecules blocked the active sites of lower energy and inhibited the mild steel dissolution. The quantum chemical study showed that NAD possess a strong electron donating and accepting properties which enhanced its strong adsorption on the metal surface.
Acknowledgement
We are grateful to Tertiary Education Trust Fund (TET Fund) Nigeria for supporting this work.
References
1. Abiola O. K., Otaigbe J. O. E., The effects of Phyllanthusamarus extract on corrosion and kinetics of corrosion processes of aluminium in alkaline solution, Corrosion Science, 2009, 51, p. 2790-2793.
2. Abiola O. K., Otaigbe J. O. E., Adsorption behavior of 1-phenyl-3-methylpyrazol-5-one on mild steel from HCl solution, Int. J. Electrochem. Sci., 2008, 3, p. 191-198.
3. Obot I. O., Obi-Egbedi N. O., Inhibitive properties, thermodynamic and quantum chemical studies of alloxazine on mild steel corrosion in H2SO4, Corrosion Science, 2011, 53, p. 263-275.
4. Arukalam I. O., Madufor I. C., Ogbobe O., Oguzie E., Experimental and Theoretical Studies of Hydroxyethyl Cellulose as Inhibitor for Acid Corrosion Inhibition of Mild Steel and Aluminium, The Open Corrosion Journal, 2014, 6, p. 1-10.
5. Solmaz R., Investigation of corrosion inhibition mechanism and stability of Vitamin B1 on mild steel in 0.5 M HCl solution, Corrosion Science, 2014, 81, p. 75-84.
6. Ameh P. O., Koha P. U., Eddy N. O., Experimental and Quantum Chemical Studies on the Corrosion Inhibition Potential of Phthalic Acid for Mild Steel in 0.1 M H2SO4, Chemical Science Journal, 2015, 6, 3, p. 1-8.
7. Oguzie E. E., Enenebeaku C. K., Akalezi C. O., Okoro S. C., Ayuk A. A., Adsorption and corrosion-inhibiting effects of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media, J. Colloid interface Sci., 2010, 349, p. 283-292.
8. Naguib A. M., Mahross M. H., Khalil H. F. Y., Mahran B. N. A., Yehia M. M., El-Sabbah M. M. B., Azo Dye Compounds as Corrosion Inhibitors for Dissolution of Mild Steel in Hydrochloric Acid Solution, Portugaliae Electrochimica Acta, 2013, 31(2), p. 119-139.
9. Mabrouk E. M., Eid S., Attia M. M., Corrosion inhibition of carbon steel in acidic medium using azo chromotropic acid dye compound, Journal of Basic and Environmental Sciences, 2017, 4, p. 351-355.
10. Oyeneyin O. E., Structural and Solvent Dependence of the Electronic Properties and Corrosion Inhibitive Potentials of 1,3,4-thidiazole and Its Substituted Derivatives - A Theoretical Investigation, Physical Science International Journal, 2017, 16(2), p. 1-8.
11. Kara Y. S., Seda G.S., Esme A., Theoretical Study on the Relationship between the Molecular Structure and Corrosion Inhibition Efficiency of Long Alkyl Side Chain Acetamide and Isoxazolidine Derivatives, Protection of Metals and Physical Chemistry of Surfaces, 2012, 48(6), p. 710-721.
12. Fouda A. S., El-Azaly A. H., Awad R. S., Ahmed A. M., New Benzonitrile Azo Dyes as Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid Solutions, International Journal of Electrochemical Science, 2014, 9, p. 1117-1131.
13. Elmsellem H., Aouniti A., Khoutoul M., Chetouani A., Hammouti B., Banchat N., Touzani R., Elazzouzi M., Theoretical approach to the corrosion inhibition efficiency of some pyrimidine derivatives using DFT method of mild steel in HCl solution, Journal of Chemical and Pharmaceutical Research, 2014, 6(4), p. 1216-1224.
14. Hasanov, R., Bilge, S., Bilgiç, S., Gece, G. and Kılıç, Z. Experimental and theoretical calculations on corrosion inhibition of steel in 1 M H2SO4 by crown type polyethers. Corrosion Science, 2010, 52(3), p. 984-990.
15. Li X., Xie X., Deng S., Du G., Inhibition effect of two mercaptopyrimidine derivatives on cold rolled steel in HCl solution, Corrosion Science, 2015, 92, p. 136-147.
16. Mwadham M. K., Lutendo C. M., Eno E. E. Theoretical Studies on Phenazine and Related Compounds as Corrosion Inhibitors for Mild Steel in Sulphuric Acid Medium, Int. J. Electrochem. Sci., 2012, 7, p. 7179-7205.
17. Lukovits I., Kalman E., Zucchi F., Corrosion Inhibitors: Correlation between the electronic structures and efficiency, Corrosion Science, 2001, 57, 1, p. 3-8.
18. Zhang F., Tang Y., Cao Z., Jing W., Wu Z., Chen Y., Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid, Corrosion Science, 2012, 61, p. 1-9.
19. Geethamani P., Kasthuri P. K., Adsorption and corrosion inhibition of mild steel in acidic media by expired pharmaceutical drug, Cogent Chemistry, 2015, 1(1), 1091558.
20. Botros R., U.S. Patent No. 4,051,119. Washington, DC: U.S. Patent and Trademark Office, 1977.
21. Ahmadi R. A., Amani S., Synthesis, spectroscopy, thermal analysis, magnetic properties and biological activity studies of Cu (II) and Co (II) complexes with Schiff base dye ligands, Molecules, 2012, 17(6), p. 6434-6448.
22. Li X. H., Deng S. D., Fu H., Inhibition by Jasminum nudiflorumLindl leaves extract of the corrosion of cold rolled steel in hydrochloric acid solution, J. Appl. Electrochem. 2010, 40, p. 1641-1649.
23. Deng S., Li X., Inhibition by Jasmium nudiflorum Lindl. Leaves extract of the corrosion of aluminium in HCl solution, Corrosion Science, 2012, 64. p. 253-262.
24. Mu G. N., Li X. H., Qu Q., Zhou J., Molybdate and tungstate as corrosion inhibitors for cold rolling steel in hydrochloric acid solution, Corrosion Science, 2006. 48, p. 445-459.
25. Stoyanova A. E., Sokolova E. I., Raicheva S. N., The inhibition of mild steel corrosion in 1 M HCl in the presence of linear and cyclic thiocarbamides-Effect of concentration and temperature of the corrosion medium on their protective action, Corrosion Science, 1997, 39, p. 1595-1604.
26. Fiori-Bimbi M. V., Alvarez P. E., Vaca, H., Gervasi, C. A., Corrosion inhibition of mild steel in HCL solution by pectin, Corrosion Science, 2015, 92, p. 192-199.
27. El-Lateef, H. M. A., Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions, Corrosion Science, 2015, 92, p. 104-117.
28. Tu S., Jiang X., Zhou L., Wang H., Jiang X., Synthesis of N-alkyl-4-(4-hydroxybut-2-ynyl) pyridinium bromides and their corrosion inhibition activities on X70 steel in 5M HCl, Corrosion Science, 2012, 65, p. 13-25.
29. Chen W., Luo H. Q., Li N. B., Inhibition effects of 2, 5-dimercapto-1, 3, 4-thiadiazole on the corrosion of mild steel in sulphuric acid solution, Corrosion Science, 2011, 53(10), p. 3356-3365.
30. Obot I. B., Obi-Egbedi N. O., Fluconazole as an inhibitor for aluminium corrosion in 0.1 M HCl, Colloids and Surfaces a: Physicochemical and Engineering Aspects, 2008, 330(2), p. 207-212.
31. Szauer T., Brandt A., Adsorption of oleates of various amines on iron in acidic solution, Electrochimica Acta, 1981, 26(9), p. 1253-1256.
32. Dahmani M., Et-Touhami A., Al-Deyab S. S., Hammouti B., Bouyanzer A., Corrosion inhibition of C38 steel in 1M HCl: A comparative Study of Black Pepper Extract and its isolated piperine, International Journal of Electrochemical Science, 2010, 5, p. 1060-1069.
33. Peme T., Olasunkanmi L. O., Bahadur I., Adekunle A. S., Kabanda M. M., Ebenso E. E., Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: gravimetric, electrochemical, quantum chemical studies and synergistic effect with iodide ions, Molecules, 2015, 20(9), p. 16004-16029.
34. Saliyan R., Adhikari A. V., Inhibition of corrosion of mild steel in acid media by N'-benzylidene-3-(quinolin-4-ylthio) propanohydrazide, Bulletin of Materials Science, 2008, 31(4), p. 699-711.
35. Chen Z. Y., Li L. J., Zhang G. A., Qiuand Y. B., Guo X. P., Inhibition effect of propargylalcohol on the stress corrosion cracking of super 13Cr steel in a completion fluid, Corros. Sci., 2013, 69, p. 205-210.
36. Hu J. Y., Zeng D. Z., Zhang Z., Shi T. H., G.L. Song G. L., Guo X. P., 2-Hydroxy-4-methoxy-acetophenone as an environment-friendly corrosion inhibitor for AZ91D magnesium alloy, Corros. Sci., 2013, 74, p. 35-43.
37. Al-Amiery A. A., Kadhum A. A. H., Kadihum A., Mohamad A. B., How C. K., Junaedi S., Inhibition of Mild Steel Corrosion in Sulfuric Acid Solution by New Schiff Base, Materials, 2014, 7, p. 787-804.