2-aminoethylthiol substituted cobalt and manganese phthalocyanines: spectroscopic and electrochemical investigations
Owolabi Mutolib BANKOLE
Chemical Sciences Department, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
E-mail: bankolemutolib@yahoo.com
Abstract
Spectroscopic and electrochemical properties of
4-(2-aminoethylthio phthalocyanato complexes of cobalt (complex 3) and
manganese (complex 4) were investigated. The UV-Vis spectral properties
of the complexes were typical of the nature of central metal (cobalt versus
manganese) phthalocyanine. The Q-band of manganese 4-(2-aminoethylthio)
phthalocyanine is red shifted with respect to cobalt manganese 4-(2-aminoethylthio)
phthalocyanine. Solution electrochemistry of complex 3 showed three
distinctly resolved redox processes attributed to ![]() (
(![]() ),
), ![]() (
(![]() ) and
) and ![]() /
/ ![]() (
(![]()
![]() ). No ring based oxidation was observed in complex 3. Complex
4 showed both ring-based oxidation, attributed to
). No ring based oxidation was observed in complex 3. Complex
4 showed both ring-based oxidation, attributed to ![]() (
(![]()
![]() ) and ring-based reduction associated with
) and ring-based reduction associated with ![]() (
(![]()
![]() ), with the normal metal reduction,
), with the normal metal reduction, ![]() /
/![]() (
(![]()
![]() ). The cathodic to anodic differences (E
). The cathodic to anodic differences (E![]() ) of redox processes observed for 4 are 180mV
) of redox processes observed for 4 are 180mV![]() and 130 mV
and 130 mV![]() while the cathodic to anodic peak difference (E
while the cathodic to anodic peak difference (E![]() ) for 3 are 290 mV
) for 3 are 290 mV![]() and 210 mV
and 210 mV ![]() at processes I and II. By virture of this, manganese
4-(2-aminoethylthio) phthalocyanine complex has better electron transfer
processes than the cobalt analogue.
at processes I and II. By virture of this, manganese
4-(2-aminoethylthio) phthalocyanine complex has better electron transfer
processes than the cobalt analogue.
Keywords
Phthalocyanine; Electrochemistry; Spectroscopic; Manganese phthalocyanine; Cobalt phthalocyanine; Electrochemical properties
Introduction
Phthalocyanines are macrocyclic compounds that are structurally related to other macrocylic pigments such as porphyrins. The main structural differences between phthalocyanines and porphyrins are [1]; (i) the presence of four isoindole subunits in phthalocyanine complex have been replaced by pyrrole units in porphyrin; (ii) presence of four nitrogen atoms at the four meso positions of phthalocyanines while the pyrrole units in porphyrin are connected via carbon atoms.
Metallophthalocyanines have shown to be extremely versatile materials with state-of-the-art applications in electro catalysis and electro analysis [1-3]. They are employed as electrocatalysts when incorporate on electrode surfaces by acting as electron transfer mediators [1-3] for various applications including as sensors, electrochromic devices, semiconductors, nonlinear optics [4-9], photosensitizers, molecular electronics, photocatalysis [10-12]. As electrocatalysts, the majority of Metallophthalocyanine (MPc) complexes involve a change in oxidation state of the central metal.
Physical, spectroscopic and electrochemical properties of phthalocyanines depend largely on the nature and position of the substituents on MPc rings, axial ligations and nature of the central transition metals [13]. Fine-tuning the spectroscopic and electrochemical properties of Pcs can be achieved by varying the central metal atoms in the cavity of the Pc ring for improved functionalities [14-17]. Electrochemical behaviors of phthalocyanines containing electroactive central metals, such as cobalt [1417] and manganese [18-20], have been reported. Their improved electron transfer processes are well documented [14-20]. Hence, phthalocyanine derivatives of Co and Mn are good candidates for the development of smart electrochemical sensors and other electrocatalysis applications [21-24]. The choice of 2-aminoethanethiol substituents for the studied complexes in this work was due to good spectroscopic, photochemical and enhanced electrochemical behaviors of sulfur-substituted phthalocyanines [25-27]. Hence, 4-(2-aminoethyl) thiol peripherally substituted Pc complexes containing Co and Mn analogues were employed in this work. This present work describes the spectroscopic and electrochemical properties of 2-aminoethylthiol substituted cobalt (3) and manganese (4) phthalocyanine complexes.
The importance of the aminoethanethiol groups on phthalocyanine complexes as potential electrocatalytic materials is showd in this study. Comparative studies on the spectroscopic and electrochemical of the two synthesized complexes are therefore reported.
Material and method
Materials
Potassium carbonate, cobalt (II)
chloride, manganese (II) acetate and dichloromethane (DCM) and
2-aminoethanethiol hydrochloride was obtained from Sigma-Aldrich.
Dimethylformamide (DMF), dimethyl sulphoxide (DMSO), methanol, ethanol, silica
gel 60 (0.04-0.063 mm) (for column chromatography) were purchased from Merck.
Tetrabutylammonium tetrafluoroborate (TBABF4![]() (electrolyte for electrochemical experiments involving MPc
complexes), 4-nitrophthalonitrile was obtained from Chemsavers. All solvents
were distilled before use and all other reagents were of analytical grade and
were used as received from the suppliers without further purification.
(electrolyte for electrochemical experiments involving MPc
complexes), 4-nitrophthalonitrile was obtained from Chemsavers. All solvents
were distilled before use and all other reagents were of analytical grade and
were used as received from the suppliers without further purification.
Instrumentation
Various spectroscopic techniques were employed for characterization of the synthesized phthalonitrile and MPc complexes. Ground state electronic absorption spectra were recorded on a Cary 500 UV-Vis-NIR spectrometer. IR spectra (KBr pellets) were recorded on a Perkin-Elmer spectrum 2000 FTIR spectrometer. 1H NMR spectra were obtained using a Bruker AVANCE 300 MHz NMR spectrometer in DMSO-d6. Elemental analyses were done using a Vario-Elementar Microcube ELIII, while mass spectra data were collected on a Bruker AutoFLEX III Smart-beam TOF/TOF mass spectrometer using a-cyano-4-hydrocinnamic acid as the matrix in the positive ion mode.
Electrochemical data were obtained using BioAnalytical systems (BAS) model 100B/W Electrochemical Workstation, or using the Autolab potentiostat PGSTAT 30 (Eco Chemie, Utretch, The Netherlands) using the General Purpose Electrochemical System data processing software (GPES, software version 4.9) or Advanced Electrochemical System (Princeton Applied Research) PAR-STAT 2273 equipment.
For all electrochemical experiments, a conventional three-electrode system was used. The working electrode was bare glassy carbon; silver-silver chloride wire and platinum wire were used as pseudo reference and auxiliary (counter) electrodes respectively.
The potential response of the Ag|AgCl![]() pseudo-reference electrode was less than the Ag|AgCl (3 M KCl)
pseudo-reference electrode was less than the Ag|AgCl (3 M KCl)![]() by 0.015±0.003V.
by 0.015±0.003V. ![]() Prior to use, the electrode surface was polished with alumina on a
Buehler felt pad and rinsed with excess Millipore water. Electrochemical
experiments involving MPc complexes were performed in freshly distilled DMF,
containing
Prior to use, the electrode surface was polished with alumina on a
Buehler felt pad and rinsed with excess Millipore water. Electrochemical
experiments involving MPc complexes were performed in freshly distilled DMF,
containing ![]() as supporting electrolyte. All electrochemical experiments were
carried out in nitrogen saturated solutions.
as supporting electrolyte. All electrochemical experiments were
carried out in nitrogen saturated solutions.
Synthesis
4-(2-aminoethylthio) phthalonitrile (2), Scheme 1
The phthalonitrile precursor (2), Scheme 1, for the preparation of the aminoethane thio-substituted Pcs (3 and 4) was prepared following the literature procedure of 4-Propy-2-ynyloxy-phthalonitrile [28]. Briefly, a mixture of 4-nitrophthalonitrile 1 (1.0 g, 5.866mmol) and 2-aminoethanethiol hydrochloride (0.667 g, 5.866mmol) was stirred in anhydrous DMF (50 ml) under nitrogen atmosphere. After stirring for 15 minutes, finely ground anhydrous K2CO3 (9.61 g, 17.38 mmol) was added portion-wise during a 2 h period, after which the reaction mixture was allowed to stir for 48 h at room temperature. The crude product was poured into ice (250 g). The precipitate was filtered off, washed several times with water, until the filtrate was neutral. The pure product obtained was air dried. Yield of the product is 0.6485g (46%). IR (KBr) obtained for 2 is as follows; vmax/cm-1: 3232.85-3358.18 (N-H), 3095.85 (Ar-C-H), 2931.9 (CH2), 2231.71 (C≡N), 1654.98, 1581.68, 1533.46, 1431.23, 1342.5, 1220.98, 1192.05, 1126.47, 1192.05, 1126.47, 1070.53, 906.57, 871.85, 835.21, 713.69, 669.32, 599.88, 524.66, 418.57. MS, m/z: calcd 203.05; found 203.11 [M]+. Anal. for C10H9N3S %: calcd C 59.09, H 4.46, N 20.67, S 15.78; found C 58.98, H 4.61, N 20.17, S 15.29. 1H NMR (300 MHz, DMSO-d6), δ (ppm): 7.78 (Ar-H,s, 1H), 7.71 (Ar-H,d, 1H), 7.40 (Ar-H,d, 1H), 3.12 (CH2, t, 2H), 2.81 (CH2, m, 2H), 2.20-2.66 (CNH2, t, 2H).
Scheme 1. Synthetic pathway for the synthesis of 4-(2-aminoethythio) phthalonitrile (2)
4-(2-aminoethylthio) phthalocyanine Cobalt (II), 3, Scheme 2
A mixture of compound 2 (0.05 g, 0.246 mmol), cobalt (II) chloride (0.032 g, 0.246 mmol), n-pentanol (5 mL) and DBU (0.5 mL) were refluxed for 20 h under nitrogen atmosphere. At the completion of the reaction, the crude deep green product was precipitated with excess MeOH:H2O (1:1) and purified by using column chromatography with neutral alumina as column material and DCM/MeOH (10:1) as eluent. The pure compound 3 was obtained as green solid. Yield: 0.08 g (38%). UV-Vis (DMF): λmax (nm) (log ε): 693 (5.3), 628 (4.8), 478 (4.2); IR (KBr) vmax/cm-1; 3232.85-3358.18 (N-H), 3095.85 (Ar-C-H), 2966-2799 (CH2), 686 (C-S-C), 1619, 1572, 1508, 1462, 1384, 1318, 1239, 1113, 919, 739. MS, m/z: calcd 871.14; found 872.44 [M+H]+. Anal. for C40H36N12S4Co %: calcd C 55.10, H 4.16, N 19.28, S 14.71; found C 55.80, H 4.82, N 18.96, S 14.22. 1H NMR (300 MHz, DMSO-d6), δ (ppm): 8.46 (Pc-H, 4H, m), 8.27, 7.95-7.71 (Pc-H, 8H, m), 3.12-2.56 (ethyl-H, 16H, m), 2.91-2.84 (CNH2, t, 8H).
4-(2-aminoethylthio) phthalocyanine Manganese (III), 4, Scheme 2
The synthesis of compounds 4 and 3 were similar, except that 4 required the use of manganese acetate as metal salt in place of cobalt II chloride. Purification was achieved using column chromatography with neutral alumina as column material and DCM/MeOH (10:1) as eluent. Yield: 0.08 g (38.09%). UV-Vis (DMF): λmax (nm) (log ε): 768 (5.4), 690 (4.8), 511 (4.5), 477 (4.5), 357 (5.1); IR (KBr) vmax/cm-1; 3191-3067 (Ar-C-H), 1064 689 (C-S-C), Mn-O stretch (877 cm-1), 1763, 1716, 1374, 1308, 1236, 1109. Anal. Calc. for C40H36N12S4Mn: C 55.35, H 4.18, N 19.36, S 14.78; Found: C 55.26, H 4.90, N 18.98, S 14.88. MS (MALDI-TOF) m/z: Calcd. 867.14; Found: 868.02 [M+H]+. 1H NMR (300 MHz, DMSO-d6), δ (ppm): 8.28 (Pc-H, 4H, m), 8.17, 7.91-7.62 (Pc-H, 8H, m), 3.03-2.76 (ethyl-H, 16H, m), 2.40-2.14 (CNH2, t, 8H).

Scheme 2. Synthetic pathway for the formation of 4-(2-aminoethylthio) phthalocyanine Cobalt (II), 3, and Manganese (III), 4
Results and discussions
Synthesis and characterization
Synthetic pathway for the preparation of the precursor 2 to complexes 3 and 4 is represented in Scheme 1. The precursor was prepared via based-catalyzed nucleophilic aromatic displacement reactions [29,30]. Cyclotetramerization process of 2 occurred in the presence of CoCl2 and Mn(CH3COO)2 to form the proposed complexes: 3 and 4 respectively. Complexes 3 and 4 were purified using column chromatography on alumina. The pure products were obtained in satisfactorily high yields and characterized by FTIR, elemental analysis, UV-Vis, 1H NMR spectroscopies and MALDI-TOF. The characterization results obtained were in good agreements with the proposed structures shown in the Scheme 2. The compounds are effortlessly soluble in in organic solvents such as DMF, DCM, THF and DMSO.
Spectroscopic properties
Molecular ion peaks of 872.44 [M+H]+ ![]() and
868.02 [M+H]+
and
868.02 [M+H]+ ![]() were
respectively observed for complexes 3 and 4. These masses are
definitive and truly represented the molecular weights of the proposed
structures. Fragmentation of m/z peaks as [M+nH]+
were
respectively observed for complexes 3 and 4. These masses are
definitive and truly represented the molecular weights of the proposed
structures. Fragmentation of m/z peaks as [M+nH]+ ![]() are in order, and have been previously reported in the literature [31].
Elemental analyses of the complexes gave percentage C, N and S values that were
within 1% in all cases, which are within acceptable range for
metallophthalocyanine complexes. 1H NMR spectra confirmed the purity
and the expected structures of the synthesized complexes. 1H NMR
spectra of the two complexes are very similar, except for the two central
metals in the Pc cavities.
are in order, and have been previously reported in the literature [31].
Elemental analyses of the complexes gave percentage C, N and S values that were
within 1% in all cases, which are within acceptable range for
metallophthalocyanine complexes. 1H NMR spectra confirmed the purity
and the expected structures of the synthesized complexes. 1H NMR
spectra of the two complexes are very similar, except for the two central
metals in the Pc cavities.
FTIR spectrum of phthalonitrile 2 shows characteristics split peaks at 3365.9 cm-1 and 3255 cm-1 attributable to N-H stretch of amine (NH2), other notable IR spectrum observed in 2 is 2231.71 cm-1 due to C≡N. Formation of complexes 3 and 4 (Figure 1) were confirmed by the disappearance of prominent nitrile (C≡N) band of compound 2. The split absorption signals around 3340-3202 cm-1 are consistent in the spectrum of the phthalonitrile as well as the synthesized molecules 3 and 4. The positions of the other absorption peaks for the synthesized complexes in the infrared spectra were all in agreement with the proposed structures and literature data [32,33].
The electronic absorption spectra of cobalt and manganese phthalocyanines showed monomeric behaviour in DMF (Figure 2); this is evidenced by single Q band for both complexes, typical of metallated phthalocyanines [34], with D4h symmetry. The Q-bands of 3 and 4 were observed at 673 nm and 768 nm, respectively. Manganese analogue was red-shifted compared to that of cobalt analogue.
The shift to higher wavelength of Q-band in compound 4 is characteristics of manganese phthalocyanines (MnPcs). This explained why MnPcs have purple colour in most cases. The position of Q-band absorption phthalocyanine complexes can be influenced by the nature of central metal, solvent, point of substitution of substituents and nature of the substituents on Pc rings [35,36]. Any of these parameters can as well be used to tune the photophysical and electrochemical properties of phthalocyanines for improved activities. Summary of absorption band maxima of complexes 3 and 4 are shown in Table 1.
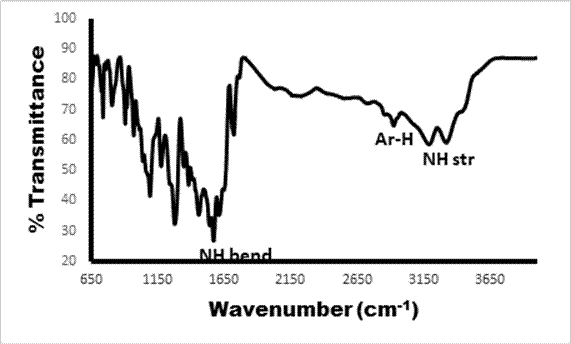
Figure 1. FTIR spectrum representative of compound 4
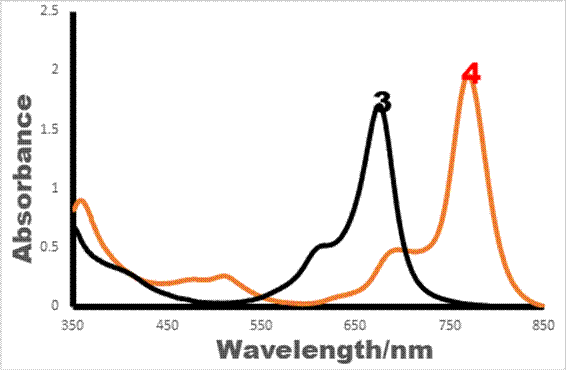
Figure 2. UV-Vis spectra of complexes 3 (2.41×10-6 M) and 4 (2.42×10-6 M) in DMF
Table 1. Summary of absorption band maxima of Manganese 4-(2-aminoethylthio) phthalocyanine and Cobalt 4-(2-aminoethylthio) phthalocyanine complexes
|
Complex |
Q-band/nm |
Vibrionic band/nm |
Charge Transfer Band/nm |
B-band/nm |
Solvent |
|
3 |
673 |
608 |
400 |
350 |
DMF |
|
4 |
768 |
687 |
506 and 466 |
356 |
DMF |
Electrochemical properties of cobalt and manganese 4-(2-aminoethylthio) phthalocyanine
Cobalt 4-(2-aminoethylthio) phthalocyanine complexes, 3
Figure 3 shows cyclic voltammetry profile of 1×10-4 M![]() of cobalt 4-(2-aminoethylthio) phthalocyanine complex, in freshly distilled,
dried DMF, containing 0.1 M TBABF4 (supporting electrolyte). Three
distinctly defined redox processes can be identified as follows;
of cobalt 4-(2-aminoethylthio) phthalocyanine complex, in freshly distilled,
dried DMF, containing 0.1 M TBABF4 (supporting electrolyte). Three
distinctly defined redox processes can be identified as follows;
Process I: The process I has a half wave potential (![]() ) and the potential peak difference (
) and the potential peak difference (![]() ) is 290 mV. The process is a quasi-reversible
process due to the weak return peaks and is usually assigned to ring reduction,
) is 290 mV. The process is a quasi-reversible
process due to the weak return peaks and is usually assigned to ring reduction, ![]() couple.
couple.
Process II: The cathodic to anodic peak current (![]() ) ratio is near unity, but quasi-reversible, because of peak to peak
separation (
) ratio is near unity, but quasi-reversible, because of peak to peak
separation (![]() ) of 210 mV. This
process is usually assigned to metal reduction,
) of 210 mV. This
process is usually assigned to metal reduction, ![]() (
(![]() ).
).
Process III: this process is assigned to metal oxidation, ![]() (
(![]() ). This process is virtually irreversible due to the absence of
return peak. No ring oxidation was observed as shown in the figure 3 below.
This may be explained in terms of the electron-releasing nature of the
sulfur-containing substituents on the ring [37,38]. Since
). This process is virtually irreversible due to the absence of
return peak. No ring oxidation was observed as shown in the figure 3 below.
This may be explained in terms of the electron-releasing nature of the
sulfur-containing substituents on the ring [37,38]. Since ![]() can be observed in coordinating solvents [39], its metal oxidation
is expected [40].
can be observed in coordinating solvents [39], its metal oxidation
is expected [40].
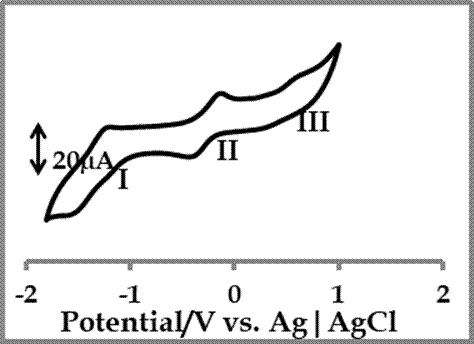
Figure
3. Cyclic
voltammetry profiles of 1×10-4
M ![]() of cobalt 4-(2-aminoethylthio)
phthalocyanine in freshly distilled DMF containing 0.1 M TBABF4
supporting electrolyte. Scan rate: 100mVs-1
of cobalt 4-(2-aminoethylthio)
phthalocyanine in freshly distilled DMF containing 0.1 M TBABF4
supporting electrolyte. Scan rate: 100mVs-1![]()
The mechanism occurring in the processes I, II and III for of cobalt 4-(2-aminoethylthio) phthalocyanine in DMF are shown in Figure 4 below:
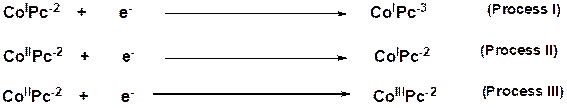
Figure 4. Redox processes I, II and III for Cobalt 4-(2-aminoethylthio) phthalocyanine complex
Manganese 4-(2-aminoethylthio) phthalocyanine complex, 4
Figure 4 shows cyclic voltammetry of 1 × 10-4 M of manganese 4-(2-aminoethylthio phthalocyanine complex, in freshly distilled, dried DMF, containing 0.1 M TBABF4 (supporting electrolyte). Three distinctly defined redox processes can be identified as follows;
Process I: The half wave potential (![]() ) and the potential peak difference (
) and the potential peak difference (![]() ) is 180 mV
) is 180 mV![]() . The process I is a quasi-reversible
with weak anodic component and large peak separation (
. The process I is a quasi-reversible
with weak anodic component and large peak separation (![]() 180 mV ). This
process is usually assigned to ring reduction,
180 mV ). This
process is usually assigned to ring reduction, ![]() couple. The cathodic to anodic peak
current (
couple. The cathodic to anodic peak
current (![]() ) ratio is 0.096. The split observed in the voltammogram of process I
could be due to aggregation [41] since peripherally substituted ligand have
a stronger tendency to aggregate in the solvent [42-44].
) ratio is 0.096. The split observed in the voltammogram of process I
could be due to aggregation [41] since peripherally substituted ligand have
a stronger tendency to aggregate in the solvent [42-44].
Process II: the anodic peak potential (![]() ) and cathodic peak potential (
) and cathodic peak potential (![]() ) in process II are 0.02V
) in process II are 0.02V ![]() and -0.15V
and -0.15V![]() respectively. The cathodic to anodic peak current (
respectively. The cathodic to anodic peak current (![]() ) ratio is near unity (0.87), but quasi-reversible, because of peak
to peak separation (
) ratio is near unity (0.87), but quasi-reversible, because of peak
to peak separation (![]() ) of 130 mV. This process is usually assigned to metal reduction, MnIIIPc-2/MnIIPc-2
) of 130 mV. This process is usually assigned to metal reduction, MnIIIPc-2/MnIIPc-2
![]() .
.
Process III: this process is assigned to weak ring-based oxidation, MnIIIPc-1/MnIIIPc-2
(E1/2 = ![]() ) consistent with literature [37,44,45]. This process is virtually
irreversible due to the absence of return peak.
) consistent with literature [37,44,45]. This process is virtually
irreversible due to the absence of return peak.
The redox processes I, II and III for manganese 4-(2-aminoethylthio) phthalocyanine complex are shown in Figure 5.
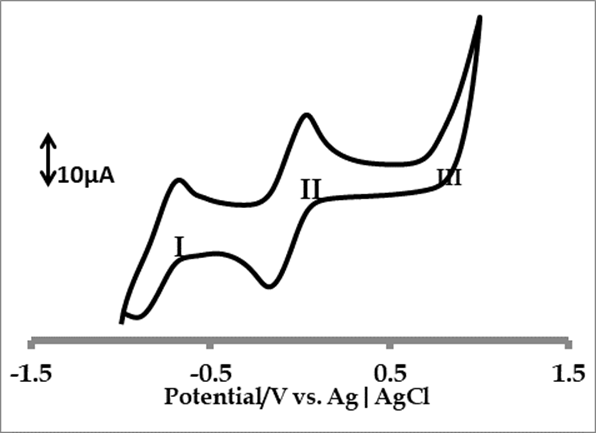
Figure 4.
Cyclic
voltammetry profiles of 1 × 10-4
M ![]() of Manganese 4-(2-aminoethylthio)
phthalocyanine complex in freshly distilled DMF containing 0.1 M TBABF4
supporting electrolyte. Scan rate: 100 mVs-1
of Manganese 4-(2-aminoethylthio)
phthalocyanine complex in freshly distilled DMF containing 0.1 M TBABF4
supporting electrolyte. Scan rate: 100 mVs-1![]()
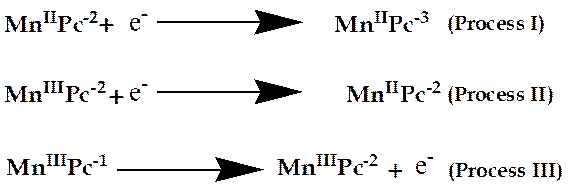
Figure 5. Redox processes I, II and III for Manganese 4-(2-aminoethylthio) phthalocyanine complex
Table 2. Summary of half wave potential
(![]() ) data of MnPc and CoPc complexes in DMF, in
) data of MnPc and CoPc complexes in DMF, in ![]()
|
Complex |
|
|
|
|
|
|
3 |
– 1.40 V |
-0.275 V |
|
+0.64 V |
|
|
4 |
|
|
– 0.80 V |
-0.07 V |
+0.86 V |
|
3 = Cobalt 4-(2-aminoethylthio) phthalocyanine, 4 = Manganese 4-(2-aminoethylthio) phthalocyanine |
|||||
The more positive the value of half
wave potential (![]() ) of an electroactive specie, the easier it gets to be reduced
during cyclic voltammetry techniques. From the above (Table 2), process
III of manganese 4-(2-aminoethylthio) phthalocyanine has higher positive value of half
wave potential (
) of an electroactive specie, the easier it gets to be reduced
during cyclic voltammetry techniques. From the above (Table 2), process
III of manganese 4-(2-aminoethylthio) phthalocyanine has higher positive value of half
wave potential (![]() ) than that of cobalt 4-(2-aminoethylthio) phthalocyanine (
) than that of cobalt 4-(2-aminoethylthio) phthalocyanine (![]() ), hence, reduction in process III of MnPc is energetically feasible
than in process III of CoPc.
), hence, reduction in process III of MnPc is energetically feasible
than in process III of CoPc.
The processes I (-1.40V![]() ) and II (-0.275V
) and II (-0.275V![]() ) of cobalt 4-(2-aminoethylthio) phthalocyanine are more negative
than that of manganese 4-(2-aminoethylthio) phthalocyanine. By virtue of this,
oxidation processes I and II in cobalt 4-(2-aminoethylthio) phthalocyanine are
more energetically feasible than that of manganese 4-(2-aminoethylthio) phthalocyanine.
) of cobalt 4-(2-aminoethylthio) phthalocyanine are more negative
than that of manganese 4-(2-aminoethylthio) phthalocyanine. By virtue of this,
oxidation processes I and II in cobalt 4-(2-aminoethylthio) phthalocyanine are
more energetically feasible than that of manganese 4-(2-aminoethylthio) phthalocyanine.
Conclusions
The effect of central transition metals; (cobalt versus manganese) was evident on their ground state absorption behaviours as seen in the UV-Vis spectra. The observed Q-band of manganese 4-(2-aminoethylthio) phthalocyanine (4) was red shifted with respect to cobalt 4-(2-aminoethylthio) phthalocyanine (3). The detailed structural characterizations of the complexes were in good agreements with the expected results.
Both manganese and cobalt phthalocyanine complexes
synthesized showed interesting electrochemical properties consistent with that
reported for 3 and 4 complexes. The cathodic to anodic peak
differences (![]() ) of redox processes
observed for 4 are 180 mV
) of redox processes
observed for 4 are 180 mV![]() and 130 mV
and 130 mV![]() , while the cathodic to anodic peak differences ((
, while the cathodic to anodic peak differences ((![]() ) for 3 are 290 mV and 2010 mV
) for 3 are 290 mV and 2010 mV![]() at processes I and II. By virtue of this, manganese
phthalocyanine (4) has the best electron transfer processes than cobalt
phthalocyanine (3) due to relatively smaller cathodic to anodic peak
differences (
at processes I and II. By virtue of this, manganese
phthalocyanine (4) has the best electron transfer processes than cobalt
phthalocyanine (3) due to relatively smaller cathodic to anodic peak
differences (![]() ) of the observed
redox processes. The sluggish electron transfer processes in 3 are due
to the relatively larger cathodic to anodic peak differences.
) of the observed
redox processes. The sluggish electron transfer processes in 3 are due
to the relatively larger cathodic to anodic peak differences.
References
1. Nombona N., Electrochemical Studies of Titanium, Manganese and Cobalt Phthalocyanines, PhD Thesis, Rhodes University, 2009.
2. Foster C. W., Pillay, J., Metters J. P., Banks C. E., Cobalt phthalocyanine modified electrodes utilised in electroanalysis: nano-structured modified electrodes vs. bulk modified screen-printed electrodes, Sensors (Basel), 2014, 19, p. 21905-21922.
3. Griveau S., Gulppi, M., Pavez, J., Zagal, J. H., Bedioui F., Cobalt phthalocyanine-based molecular materials for the electrocatalysis and electroanalysis of 2-mercaptoethanol, 2-mercaptoethanesulfonic acid, reduced glutathione and L-cystein, Electroanalysis, 2003, 15 (9), p. 779-785.
4. Bankole O. M., Yılmaz Y., Nyokong T., Nonlinear optical behavior of alkyne terminated phthalocyanines in solution and when embedded in polysulfone as thin films: effects of aggregation, Optical Materials, 2016, 51, p. 194-202.
5. Kimura M., Ueki H., Ohta K., Hanabusa K., Shirai H., Kobayashi N., Nanoscopic fibrous assemblies made of methalophthalocyanine-terminated amphiphilic polymers, Chemistry- A European Journal, 2004, 10, p. 4954-4959.
6. Dini D., Barthel M., Hanack M., Phthalocyanines as active materials for optical limiting, European Journal of Organic Chemistry, 2001, p. 3759-3769.
7. Bonnett R., In Chemical Aspects of Photodynamic Therapy, Gordon and Breach Science Publishers, Amsterdam, 2000.
8. Huang Z., Huang J., Chen N., Huang J., Synthesis, characterization and properties of some metallophthalocyanine complexes substituted by N-piperidineethanol, Journal of Coordination Chemistry, 2008, 61 (14), p. 2315-2324.
9. Kaya E. N., Tuncel S., Basova T. V., Banimuslem H., Hassan A., Gurek A. G., Ahsen V., Durmus M., Effect of pyrene substitution on the formation and sensor properties of phthalocyanine-single walled carbon nanotube hybrids, Sensors and Actuators B, 2014, 199, p. 277-283.
10. Lukyanets E. A., Phthalocyanines as photosensitizers in the photodynamic therapy of cancer, Journal of Porphyrins and Phthalocyanines, 1999, 3, p. 424-432.
11. Dini D., Yang G. Y., Hanack M., Perfluorinated phthalocyanines for optical limiting: evidence for the direct correlationbetween substituent electron withdrawing character and the nonlinear optical effect, Journal of Chemical Physics, 2003, 119, p. 4857-4864.
12. Rodrıguez-Mendez M. L., Souto J., de Saja J. A., Aroca R., Electrochromic display based on Langmuir-Blodgett films of praseodymium bisphthalocyanine, Journal of Material Chemistry, 1995, 5, p. 639-642.
13. McKeown N.B., in: K.M. Kadish, K.M. Smith, R. Guilard (Eds.), The Porphyrin Handbook: Physical Properties of Phthalocyanine based Materials, Academic Press, New York, 2003.
14. Tasso T. T., Furuyama T., Kobayashi N., Absorption and electrochemical properties of cobalt and iron phthalocyanines and their quaternized derivatives: aggregation equilibrium and oxygen reduction electrocatalysis, Inorganic Chemistry, 2013, 52(16), p. 9206-9215.
15. Fang W., Feng W., Shi C., Xinping Q., LiQuan C., A comparison of iron phthalocyanine and cobalt porphyrin on the electrochemical catalysis in Ni-MH battery, Chinese Science Bulletin, 2007, 52 (1), p. 71-77.
16. Randin J. P., Interpretation of the relative electrochemical activity of various metal phthalocyanines for the oxygen reduction reaction, Electrochimica Acta, 1974, 19 (2), p. 83-85.
17. Rollmann L. D., Iwamoto R. T., Electrochemistry, electron paramagnetic resonance, and visible spectra of cobalt, nickel, copper, and metal-free phthalocyanines in dimethyl sulfoxide, Journal of the American Chemical Society, 1968, 90(6), p. 1455-1463.
18. Santos L. S. S., Landers R., Gushikem Y., Application of manganese (II) phthalocyanine synthesized in situ in the SiO2/SnO2 mixed oxide matrix for determination of dissolved oxygen by electrochemical techniques, Talanta, 2011, 85, p. 1213-1216.
19. Blakemore J. D., Hull J. F., Crabtree R. H., Brudvig G. W., Aqueous speciation and electrochemical properties of a water soluble manganese phthalocyanine complex, Dalton Transactions, 2012, 41, p. 7681-7688.
20. Lever A. B. P., Minor P. C., Wilshire J. P., Electrochemistry of manganese phthalocyanine in nonaqueous media, Inorganic Chemistry, 1981, 20(8), p. 2550-2553.
21. Caro C. A., Bedioui F., Zagal J. H., Electrocatalytic oxidation of nitrite on a vitreous carbon electrode modified with cobalt phthalocyanine, Electrochimica Acta, 2002, 47, p. 1489.
22. Komorsky-Lovric S., Voltametry of microcrystals of cobalt and manganese phthalocyanines, Journal of Electroanalytical Chemistry, 1995, 397(1-2), p. 211-215.
23. Cortes J. S., Granados S. G., Ordaz A. A., Jimenez J. A. L., Griveau S., Bedioui F., Electropolymerized manganese tetraaminophthalocyanine thin films onto platinum ultramicroelectrode for the electrochemical detection of peroxynitrite in solution, Electroanalysis, 2007, 19, p. 61-64.
24. Masa J., Ozoemena K., Schuhmann W., Zagall J. H., Oxygen reduction reaction using N4-metallomacrocyclic catalysts; fundamentals on rational catalyst design, Journal Porphyrins and Phthalocyanines, 2012, 16, p. 761-784.
25. Napier A., Hart J. P., Voltammetric and amperometric studies of selected thiols and dimethyldisulfide using a screen-printed carbon electrode modified with cobalt phthalocyanine: studies towards a gas sensor, Electroanalysis, 1996, 8(11), p. 1006-1013.
26. Sakamoto K., Ohno-Okumura E., Kato T., Soga H., Synthesis of near-infrared absorbed metal phthalocyanine with S-aryl groups at non-peripheral positions, J. Porphyrins Phthalocyanines, 2010, 14, p. 47-54.
27. Burnham P. M., Cook M. J., Gerrard L. A., Heeney M. J., Hughes D. L., Structural characterization of a red phthalocyanine, Chemical Communications, 2003, p. 2064-2065.
28. Bankole O. M., Britton J., Nyokong T., Photophysical and non-linear optical behavior of novel tetra alkynyl terminated indium phthalocyanines: Effects of the carbon chain length, Polyhedron, 2015, 88, p. 73-80.
29. Young J. G, Onyebuagu W., Synthesis and characterization of di-disubstituted phthalocyanines, The Journal of Organic Chemistry, 1990, 55(70), p. 2155-2159.
30. Leznoff C. C., Hu M., McArthur C. R., Qin Y., van Lier J. E., The syntheses of 2,9,16,23- and 1,8,15,22-tetrahydroxyphthalocyanines, Can. J. Chem., 1994, 72 (9), p. 1990-1998.
31. Tolbin A. Y., Pushkarev V. E., Nikitin G. F., Tomilova L. G., Heteroligand and heteronuclear clamshell-type phthalocyanines: selective preparation, spectral properties, and synthtetic application, Tetrahedron Letters, 2009, 69 (34), p. 4848-4850.
32. Bard A. J., Faaulkner L. R., Electrochemical Methods: Fundamentals and Applications (2 ed.). Wiley. 2000. ISBN 0-471-04372-9.
33. Nicholson R. S, Shain I., Theory of stationary electrode polarography. Single scan and clycli methods applied to reversible, irreversible, and kinetic systems, Analytical Chemistry, 1964, 36 (4), p. 706-723.
34. Stillman M. J., Nyokong T., in: C.C. Leznoff, A.B.P Lever (Eds.), Phthalocyanines: Properties and Applications, vol. 1, VCH Publishers, New York, 1989.
35. McKeown N. B., Chambrier I., Cook M. J., Synthesis and characterization of some 1,4,8,11,15,18,22,25-octa-alkyl- and 1,4,8,11,15,18-hexa-alkyl-22,25-bis(carboxypropyl)phthalocyanines), Journal Chemical Society, Perkin Transactions 1, 1990, p. 1169-1177.
36. Boston D. R., Bailar J. C., Phthalocyanine derivatives from 1,2,4,5-tetracyanobenzene or pyromellitic dianhydride and metal salts, Inorganic Chemistry, 1972, 11(7), p. 1578-1583.
37. Nyokong T., Isago H., The renaissance in optical spectroscopy of phthalocyanines and other tetraazzaporphyrins, Journal of Porphyrins and Phthalocyanines, 2004, 8, p. 1083.
38. Ozkaya A. R., Gurek A. G., Gul A., Bekaroglu A, O., Electrochemical and spectral properties of octakis(hexylthio)-substituted phthalocyanines, Polyhedron, 1997, 16(11), p. 1877-1883.
39. Lever A.B.P., Milaeva E.R., Speier G. in Phthalocyanines: Properties and Applications, C.C. Leznoff, A.B.P. Lever (eds), VCH Publishers, New York, 3, 1993.
40. Szczepaniak B., Bra̧giel P., The influence of oxygen adsorption on the electronic structure of copper phthalocyanine, Vacuum, 1995, 46(5-6), p. 465-467
41. Akinbulu I. A., Surface properties and electrocatalytic applications of metallophthalocyanines confined on electrode surfaces, PhD Thesis, Rhodes University, Grahamstown, 2010.
42. Agboola B. O., Ozoemena K.I., Nyokong T., Synthesis and electrochemical properties of benzyl-mercapto and dodecyl-mercapto tetrasubstituted manganese phthalocyanine complexes, Electrochimical Acta, 2007, 52(7), p. 2520-2526.
43. George R. D., Snow A. W., Shirk J. S., Barger W. R., The alpha substitution effect on phthalocyanine aggregation, Journal Porphyrins and Phthalocyanines, 1998, 2(1), p. 1-7.
44. Cook M. J., McMurdo J., Miles D. A., Poynter R. H., Haslam S. D., Richardson R. M., Welford K., Monolayer behaviour and Langmuir–Blodgett film properties of some amphiphilic phthalocyanines: factors influencing molecular organisation within the film assembly, Journal of Materials Chemistry, 1994, 4, p. 1205-1213.
45. Khene S., Geraldo D. A., Togo C. A., Limson J., Nyokong T., Synthesis, electrochemical characterization of tetra- and octa-substituted dodecyl-mercapto tin phthalocyanines in solution and as self-assembled monolayers, Electrochimica Acta, 2008, 54(2), p. 183-191.